KESIMPTA resources
Don’t miss out on these resources to support you and your patients.
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
KESIMPTA is indicated for the treatment of adult patients with relapsing forms of multiple sclerosis with active disease defined by clinical or imaging features.1
KESIMPTA demonstrated a generally well-tolerated safety profile similar to teriflunomide, maintained up to 6 years.1–3
The safety profile of KESIMPTA versus teriflunomide was assessed in the double-blind ASCLEPIOS I and II trials.1,2
Patients enrolled in ASCLEPIOS I and II were eligible for entry into an open-label, long-term safety extension (ALITHIOS).3–5
On this page, you can find data for specific AEs associated with KESIMPTA versus teriflunomide, such as infection rates and injection-site reactions, treatment discontinuations and immunoglobulin (Ig) levels.
For full safety information, please refer to KESIMPTA Summary of Product Characteristics (SmPC) for further information.1
The most important and frequently reported adverse reactions with KESIMPTA are upper respiratory tract infections (39.4%), systemic injection-related reactions (20.6%), injection-site reactions (10.9%) and urinary tract infections (11.9%).1
Adverse events reported in the SmPC
Infections and infestations | |
|---|---|
Very common | Upper respiratory tract infections† |
Common | Oral herpes |
Immune system disorders | |
Not known | Hypersensitivity reactions§ |
General disorders and administration site conditions | |
Very common | Injection-site reactions (local) |
Injury, poisoning and procedural complications | |
Very common | Injection-related reactions (systemic) |
Gastrointestinal disorders | |
Common | Nausea, vomiting¶ |
Investigations | |
Common | Blood immunoglobulin M (IgM) decreased |
Adapted from KESIMPTA Summary of Characteristics.1
ASCLEPIOS I and II study design:2
Two double-blind, double-dummy, Phase III trials1,2
Participants had relapsing multiple sclerosis
Participants were randomised to receive subcutaneous (SC) KESIMPTA (20 mg every 4 weeks after 20 mg loading doses at Days 1, 7, and 14) (n=946) or oral teriflunomide (14 mg daily) for up to 30 months (n=936)‖
KESIMPTA (n=946) | Teriflunomide (n=936) | |
Any AEs | 791 (83.6) | 788 (84.2) |
Any serious AEs | 86 (9.1) | 74 (7.9) |
Most common AEs (≥5% of patients in any treatment group) | ||
Injection-related reactions (systemic)** | 195 (20.6) | 143 (15.3) |
Nasopharyngitis | 170 (18.0) | 156 (16.7) |
Headache | 126 (13.3) | 116 (12.4) |
Injection-site reaction (local) | 103 (10.9) | 52 (5.6) |
Upper respiratory tract infection | 97 (10.3) | 120 (12.8) |
Urinary tract infection | 97 (10.3) | 78 (8.3) |
Back pain | 72 (7.6) | 58 (6.2) |
Fatigue | 71 (7.5) | 72 (7.7) |
Influenza | 62 (6.6) | 59 (6.3) |
Nausea | 61 (6.4) | 64 (6.8) |
Blood IgM decreased | 56 (5.9) | 21 (2.2) |
Alopecia | 54 (5.7) | 138 (14.7) |
Arthralgia | 49 (5.2) | 44 (4.7) |
Diarrhoea | 49 (5.2) | 111 (11.9) |
Pain in extremity | 46 (4.9) | 66 (7.1) |
Depression | 45 (4.8) | 48 (5.1) |
Hypertension | 35 (3.7) | 55 (5.9) |
Paresthesia | 27 (2.9) | 52 (5.6) |
Data are n (%)
Adapted from Hauser SL, et al. 2020.2
Discontinuation: | KESIMPTA (n=946) | Teriflunomide (n=936) |
Due to an AE | 54 (5.7%) | 49 (5.2%) |
Due to an injection reaction (systemic) | 1 (0.1%) | NA** |
Adapted from Hauser SL, et al. 20202 and Cohen AH, et al. 2023.4
ALITHIOS study design:3
Open-label, single-arm, multi-centre extension of ASCLEPIOS I/II, APLIOS and APOLITOS trials
Objective: to assess the longer-term safety and efficacy of KESIMPTA treatment for up to 6 years in pwRMS
Efficacy population for ALITHIOS included 1882 who entered from ASCLEPIOS I/II (including continuous and switch KESIMPTA groups). Safety population for ALITHIOS includes patients from APLIOS and APOLITOS. A total of 1969 patients were included in the safety analysis
At data cut-off, September 2023, patients had experienced up to 6 years of KESIMPTA treatment
For further information on the clinical trials, please click here.
No unexpected safety signals were identified3
The most common AEs were infections (COVID-19 [34.3%], nasopharyngitis [20.6%], upper respiratory tract infection [14.9%] and urinary tract infection [14.4%])3
EAIR for malignancies did not increase over time in the overall safety population3
The overall EAIR per 100 patient-years of serious infections was consistent with Phase III ASCLEPIOS I/II trials (EAIR: 1.55) and did not increase with treatment up to 6 years despite the COVID-19 pandemic; the most common serious infections were COVID-19 (1.4%)/COVID-19 pneumonia (1.3%),†† and appendicitis (0.8%)‡‡3
ALITHIOS study design:3
Open-label, single-arm, multi-centre extension of ASCLEPIOS I/II, APLIOS and APOLITOS trials
Objective: to assess the longer-term safety and efficacy of KESIMPTA in pwRMS
Efficacy population for ALITHIOS included 1882 who entered from ASCLEPIOS I/II (including continuous and switch KESIMPTA groups). Safety population for ALITHIOS includes patients from APLIOS and APOLITOS. A total of 1969 patients were included in the safety analysis
At data cut-off, September 2023, patients had experienced up to 6 years of KESIMPTA treatment
For further information on the clinical trials, please click here.
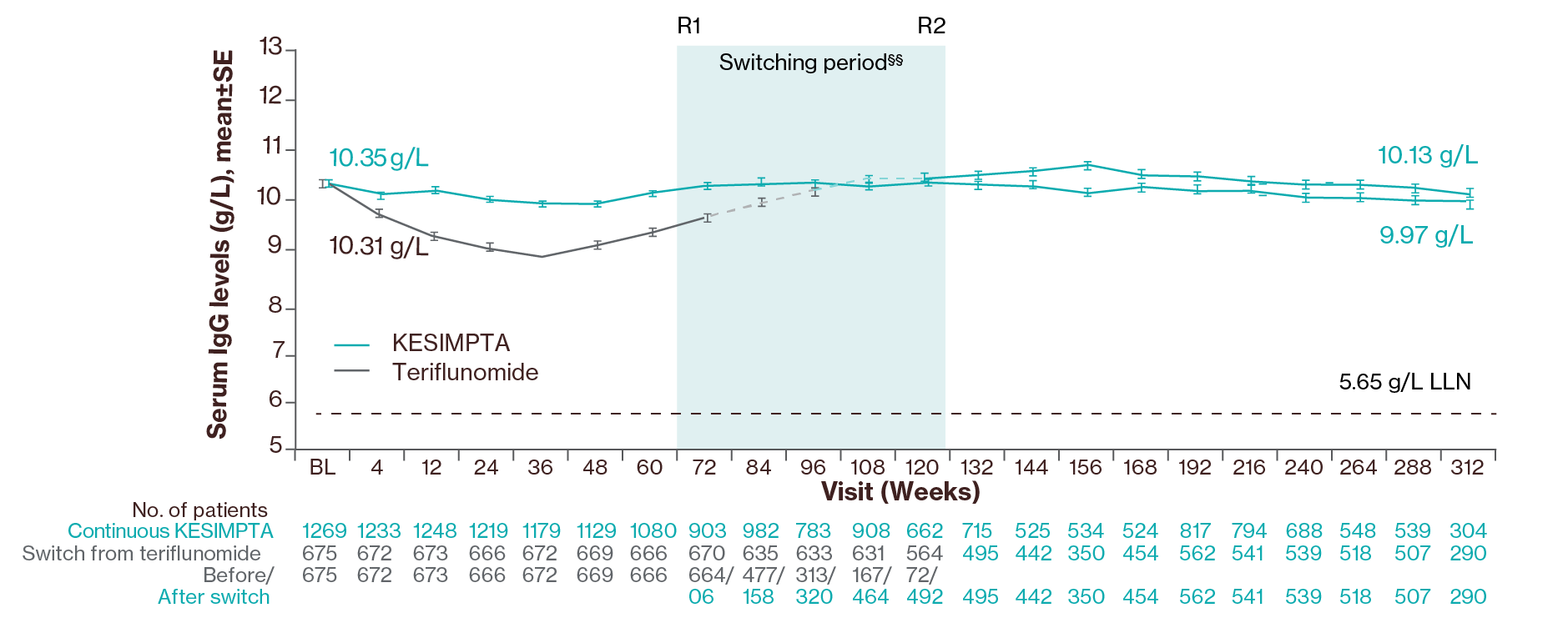
KESIMPTA was associated with a transient decrease of 4.3% in mean IgG levels after 48 weeks of treatment but an increase of 2.2% after 96 weeks.1 Mean serum IgG levels remained stable for up to 6 years of treatment and the majority of patients (97.2%) had IgG levels above the LLN.3
The most important and frequently reported adverse reactions are upper respiratory tract infections (39.4%), systemic injection-related reactions (20.6%), injection-site reactions (10.9%) and urinary tract infections (11.9%).1
Mean serum IgG levels remained generally stable and above the LLN for up to 6 years in 97% of patients (data cut-off: Sept 2023)3
Adapted from Wiendl H, et al. 2024.3
Treatment interruption/discontinuation¶¶ between treatment groups was reported in 3 (0.2%)/ 4 (0.2%) participants due to low IgG. In ASCLEPIOS I/II, the investigators were required to interrupt study treatment if IgG levels fell 20% <LLN; due to a protocol amendment at the beginning of ALITHIOS (June 3, 2021), the requirement to interrupt treatment based on a specific threshold due to low IgG/IgM was removed, and the decision was left to the discretion of the investigator.3
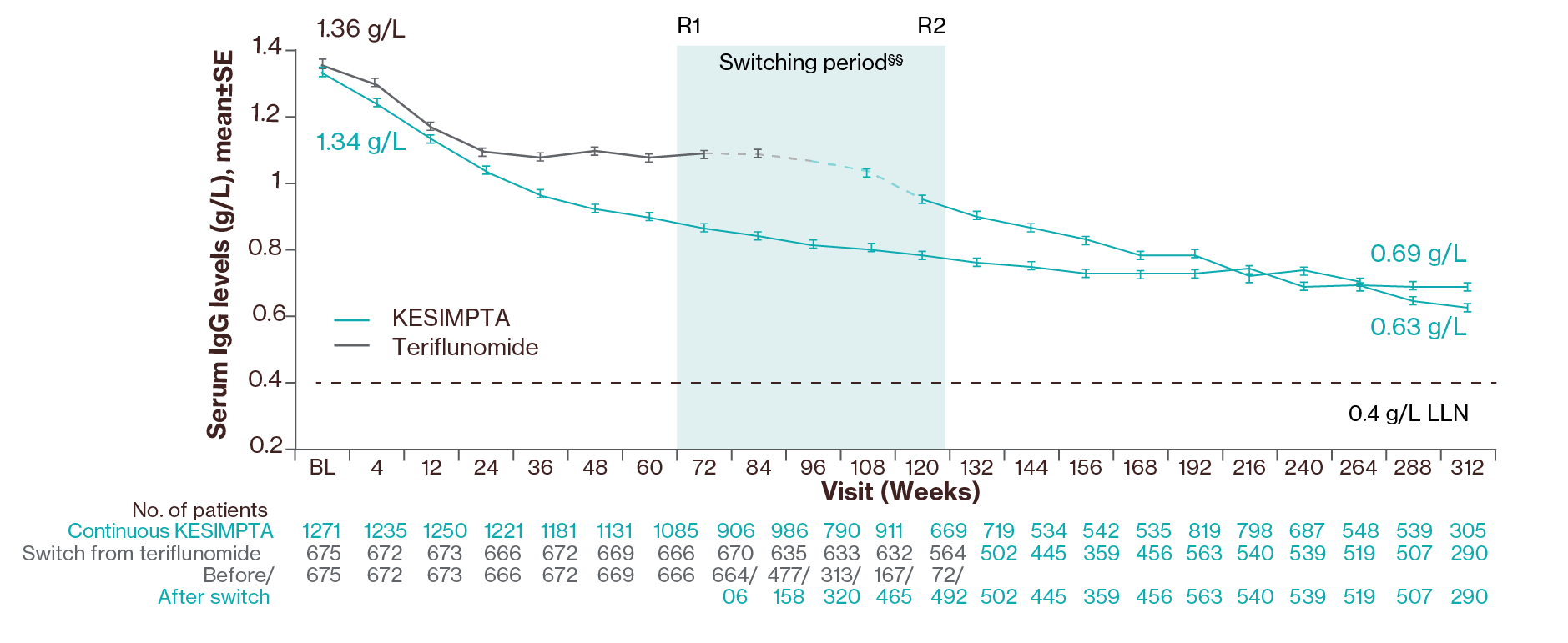
In the Phase III clinical studies, a decrease in mean value of IgM (30.9% decrease after 48 weeks and 38.8% decrease after 96 weeks) was observed and no association with risk of infections, including serious infections, was shown.1
Post-hoc analysis showed that mean IgM levels decreased in both groups but remained above the LLN for up to 6 years in the majority of the patients (66% of patients); data cut off: Sept 20233
Adapted from Wiendl H, et al. 2024.3
Treatment interruption/discontinuation¶¶ between treatment groups was reported in 203 (10.3%)/71 (3.6%) participants due to low IgM. In ASCLEPIOS I/II, the investigators were required to interrupt study treatment if IgM levels fell 10% <LLN; due to a protocol amendment at the beginning of ALITHIOS (June 3, 2021), the requirement to interrupt treatment based on a specific threshold due to low IgG/IgM was removed, and the decision was left to the discretion of the investigator.3
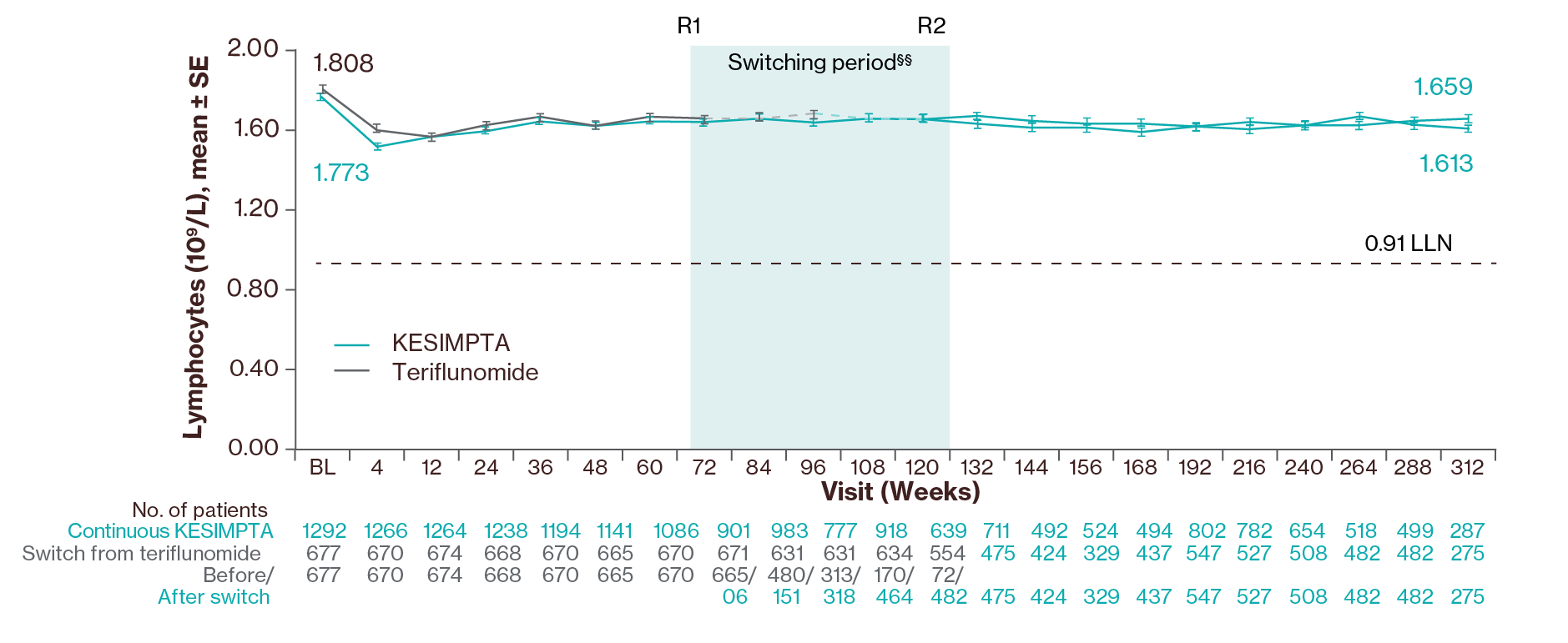
Lymphocyte levels3
Adapted from Wiendl H, et al. 2024.3
A transient decline in mean lymphocytes was observed up to Week 4 (% change: continuous, −11.9%; switch, −8.2%), followed by an increase back to close to baseline in continuous and newly switched groups through Week 312.3
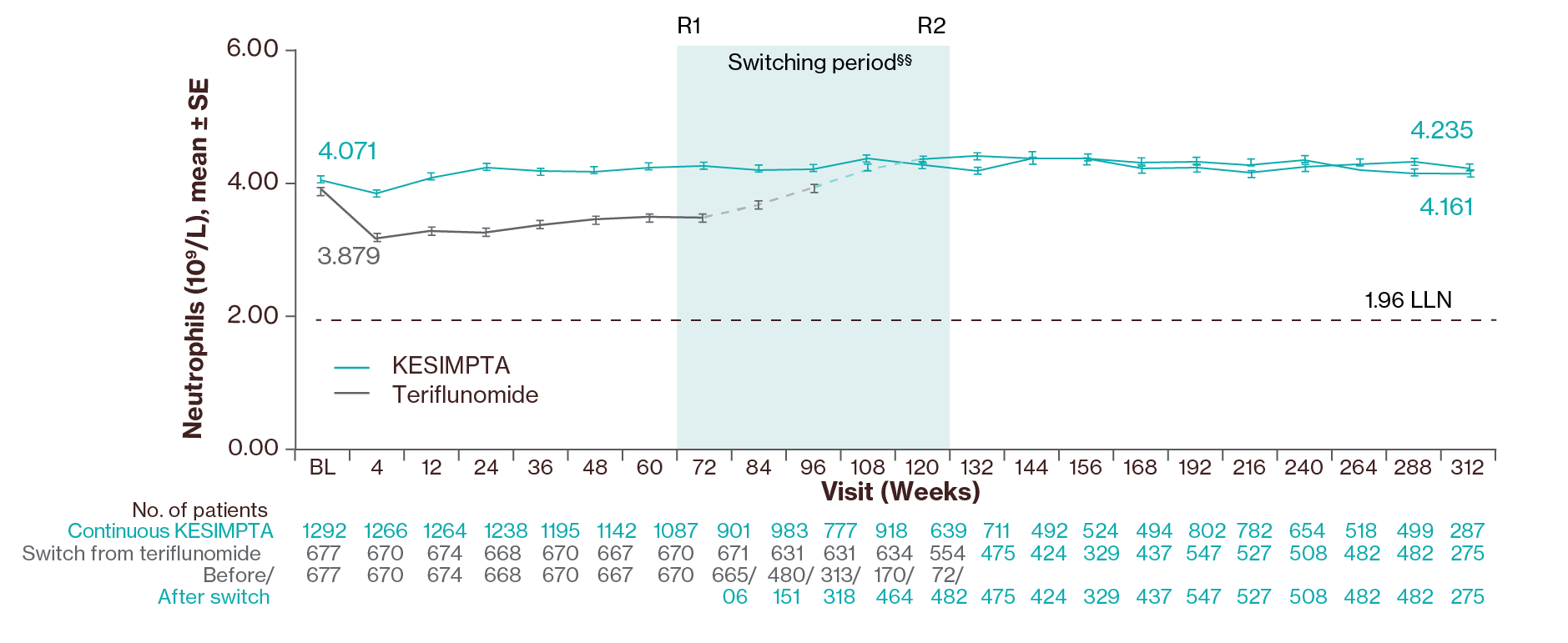
Neutrophil levels3
Adapted from Wiendl H, et al. 2024.3
Mean neutrophil levels remained stable and above baseline for all visits up to Year 6 with an increase in levels after switching from teriflunomide to KESIMPTA was observed.3
For full safety information, please refer to KESIMPTA Summary of Product Characteristics (SmPC).1
Before treatment initiation with KESIMPTA, there are a variety of prescribing considerations and precautions to consider, alongside dosing and administration guidance.
Click here for more details about clinical trial designs
Refer to the SmPC for full prescribing and safety information.1
*Very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000) and not known (cannot be estimated from the available data).1
†Grouping of preferred terms (PTs) was considered for adverse drug reaction (ADR) frequency determination and includes the following: nasopharyngitis, upper respiratory tract infection, influenza, sinusitis, pharyngitis, rhinitis, viral upper respiratory infection, tonsillitis, acute sinusitis, pharyngotonsillitis, laryngitis, pharyngitis streptococcal, viral rhinitis, sinusitis bacterial, tonsillitis bacterial, viral pharyngitis, viral tonsillitis, chronic sinusitis, nasal herpes, tracheitis.1
‡Grouping of preferred terms (PTs) was considered for ADR frequency determination and includes the following: urinary tract infection, cystitis, Escherichia urinary tract infection, asymptomatic bacteriuria, bacteriuria.1
§Reported during post-marketing experience.1
¶Nausea and vomiting have been reported in association with systemic injection-related reactions.1
‖Patients in the teriflunomide arm received a matching placebo injection to ensure blinding (double-dummy design).2
**Only reactions or symptoms that occurred within 24 hours after injection are included (i.e., time to onset of reaction ≤24 hours).2
††There are n=49 COVID-19–related SAEs in total, one of them has PT of “suspected COVID-19”; and a majority (85.71%) of the cases recovered.3
‡‡All cases of appendicitis recovered, and a majority of them were not related to KESIMPTA treatment.3
§§Switching period refers to the participants started on teriflunomide and not applicable to the participants on KESIMPTA in the core period. For the teriflunomide/KESIMPTA group, data from the first dose of teriflunomide until the last dose of KESIMPTA plus 100 days or analysis cut-off date has been used. R1: The first participant with first treatment-emergent assessment in KESIMPTA period after switching to KESIMPTA (72 weeks); R2: The last participant with last treatment-emergent assessment in teriflunomide period before switching to KESIMPTA (120 weeks). For all pooled analyses, a fixed value of LLN (using ALITHIOS study reference) was used: IgG: 5.65 g/L, IgM: 0.4 g/L, lymphocyte: 0.91x109/L and neutrophil: 1.96x109/L.3
¶¶In ASCLEPIOS I/II, the investigators were required to interrupt study treatment if IgM levels fell 10% <LLN or IgG levels fell 20% <LLN; due to a protocol amendment at the beginning of ALITHIOS (June 3, 2021), the requirement to interrupt treatment based on a specific threshold due to low IgG/IgM was removed, and the decision was left to the discretion of the investigator.3
ADR, adverse drug reaction; AE, adverse event; BL, baseline; EAIR, exposure-adjusted incidence rates; ECG, electrocardiogram; HBV, hepatitis B virus; HCP, healthcare professional; Ig, immunoglobulin; IgG, immunoglobulin G; IgM, immunoglobulin M; JCV, John Cunningham virus; LLN, lower limit of normal; MRI, magnetic resonance imaging; NA, not applicable; PML, progressive multifocal leukoencephalopathy; PT, preferred term; pwRMS, people with relapsing multiple sclerosis; RMS, relapsing multiple sclerosis; SC, subcutaneous; SmPC, summary of product characteristics.
References
KESIMPTA (ofatumumab) Summary of Product Characteristics.
Hauser SL, et al. New Engl J Med 2020;383:546–557 and supplementary material.
Wiendl H, et al. Poster P9.010. American Academy of Neurology, April 2024.
Cohen JA, et al. Poster P8.004. American Academy of Neurology (AAN), April 2023.
Clinicaltrials.gov. Long-term Safety, Tolerability and Effectiveness Study of Ofatumumab in Patients With Relapsing MS (ALITHIOS) (NCT03650114). Available at: https://clinicaltrials.gov/study/NCT03650114 [Accessed January 2025].
UK | January 2025 | 443397
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.