

This page is intended for UK healthcare professionals only.
FABHALTA® is indicated as monotherapy in the treatment of adult patients with paroxysmal nocturnal haemoglobinuria (PNH) who have haemolytic anaemia.1
FABHALTA®▼ (iptacopan)
The first and only oral PNH monotherapy that may enable improvements of Hb vs baseline1–3
FABHALTA® is indicated as monotherapy in the treatment of adult patients with paroxysmal nocturnal haemoglobinuria (PNH) who have haemolytic anaemia.1
Click here for more information on the efficacy and safety profile of FABHALTA®
Some patients with PNH may face unresolved burdens despite treatment with C5 inhibitors4–6
C5 inhibitors may lead to extravascular haemolysis (EVH), leaving patients with incomplete haemolysis control.4–6
When compared with the natural history of PNH, C5i treatment offers IVH control which may result in:
Prolonged survival4
Reduced thrombotic risk4
Reduced transfusion dependence4,7
Potential emergence of EVH in some patients on C5i therapy may mean:
50–82% of patients remain anaemic with Hb levels below 12 g/dL, as suggested by real-world evidence8
8–32% require one or more transfusions8
75–89% of patients still experience fatigue as a symptom of PNH7
Development of EVH and resulting transfusion dependence could have a negative effect on patient QoL.9
Transfusion dependence could lead to complications such as iron overload, and has been associated with lost productivity due to travel and time spent at infusion centres.9
FABHALTA® can help control both C3-mediated EVH and terminal-complement mediated IVH2
PNH is a disease caused by uncontrolled complement activity mediated by the alternative pathway.10
FABHALTA® works upstream of C5i for comprehensive targeting of haemolysis control (IVH and EVH)*1,2,11
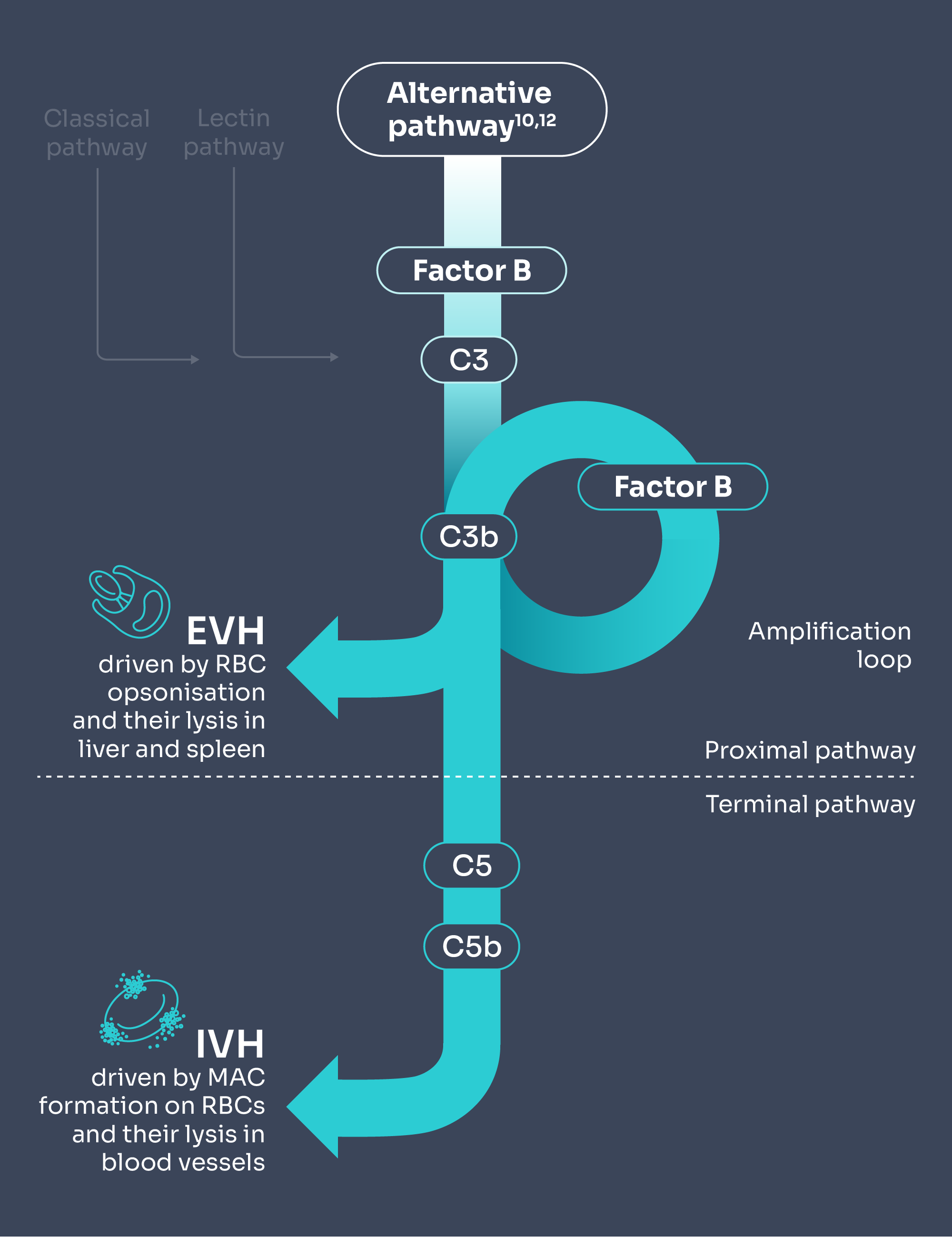
Adapted from Brodsky 2014 and Janeway et al. 2001.10,12
C5i does not control early complement activation, which could lead to some patients experiencing EVH, which could result in anaemia despite being on treatment.4–6
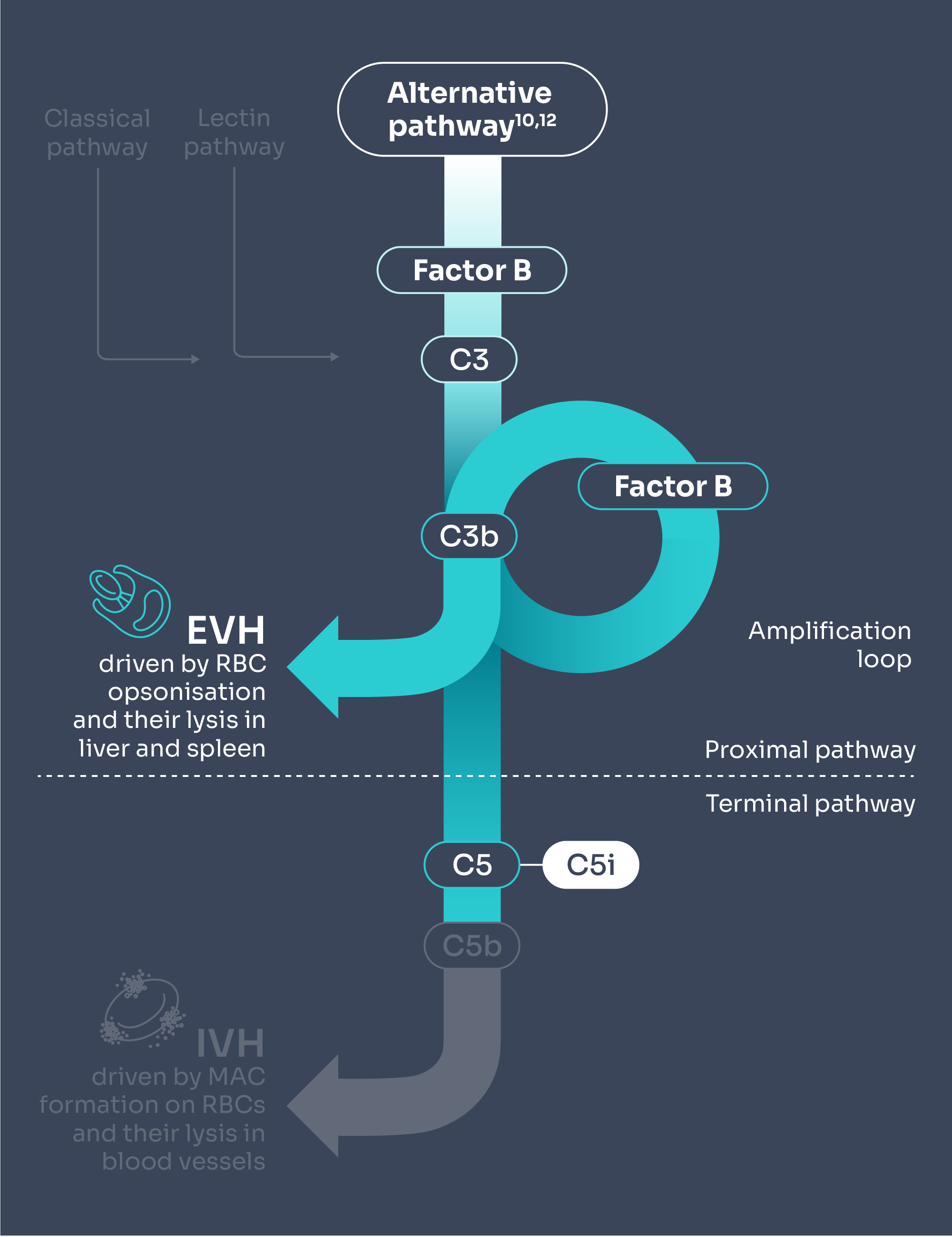
Adapted from Brodsky 2014 and Janeway et al. 2001.10,12
FABHALTA® is a proximal complement inhibitor that targets Factor B to selectively inhibit the alternative pathway of the complement cascade.1
Inhibition of Factor B prevents the activation of C3 convertase and the subsequent formation of C5 convertase to control both C3-mediated extravascular haemolysis (EVH) and terminal complement-mediated intravascular haemolysis (IVH).1
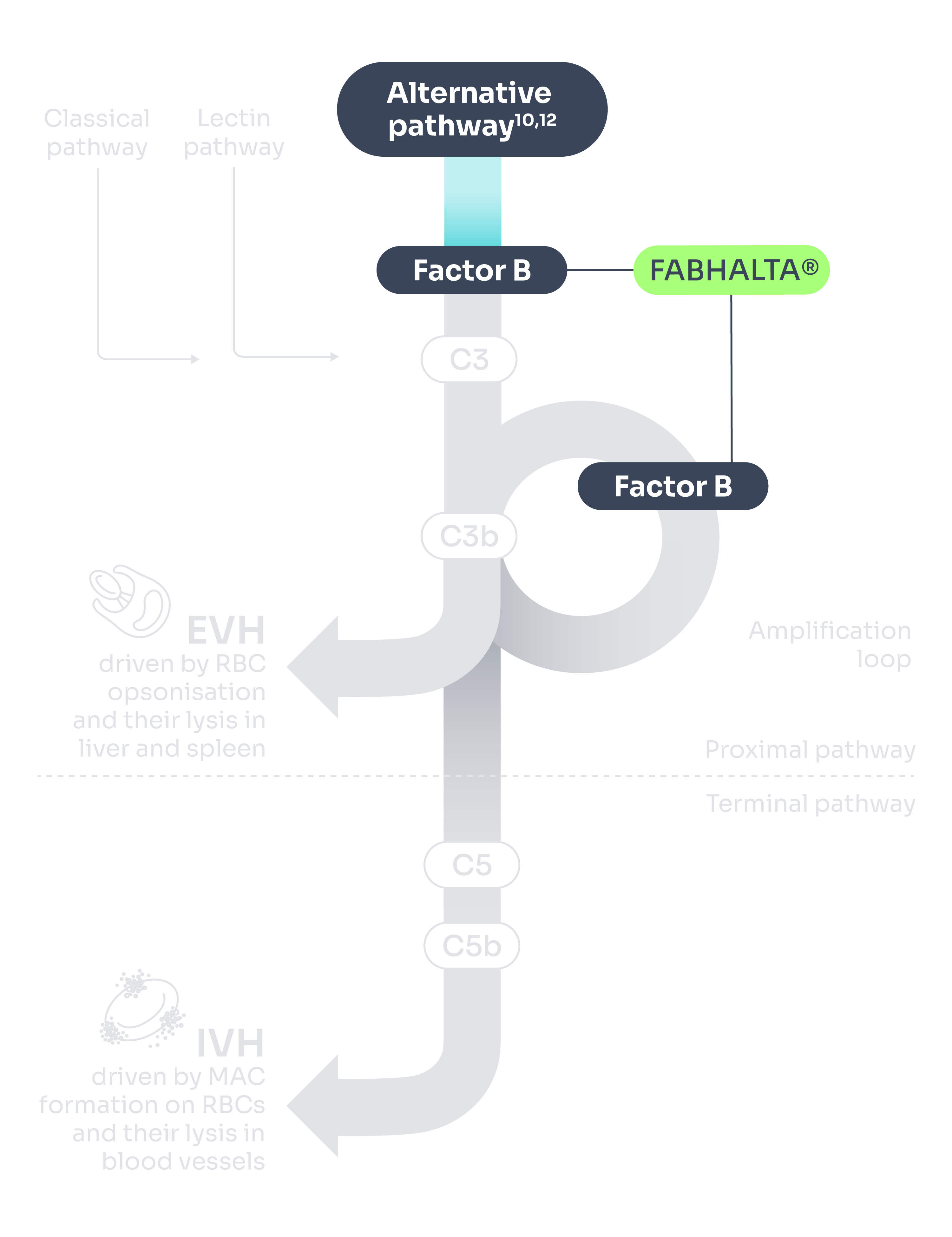
Adapted from Brodsky 2014 and Janeway et al. 2001.10,12
FABHALTA® dosing information1
The recommended dose of FABHALTA® is 200 mg taken orally twice daily.
Healthcare professionals should advise patients with PNH about the importance of adherence to the dosing schedule in order to minimise the risk of haemolysis.
If a dose or doses are missed, the patient should be advised to take one dose as soon as possible (even if it is shortly before the next scheduled dose) and then to resume the regular dosing schedule. Patients with several consecutive missed doses should be monitored for potential signs and symptoms of haemolysis.
Before starting treatment with FABHALTA®, patients must receive vaccines to reduce the risk of serious infections with encapsulated bacteria.
PNH is a disease that requires chronic treatment. Discontinuation of this medicinal product is not recommended unless clinically indicated.
Please see the FABHALTA® Summary of Product Characteristics for full information on dosing and administration and drug-drug interactions.
Patients switching from anti-C5 (eculizumab, ravulizumab) or other PNH therapies to FABHALTA®
To reduce the potential risk of haemolysis with abrupt treatment discontinuation:
For patients switching from eculizumab, FABHALTA® should be initiated no later than 1 week after the last dose of eculizumab
For patients switching from ravulizumab, FABHALTA® should be initiated no later than 6 weeks after the last dose of ravulizumab
Switches from complement inhibitors other than eculizumab and ravulizumab have not been studied.
Special populations
Elderly
No dose adjustment is required for patients 65 years of age and older.
Renal impairment
No dose adjustment is required in patients with mild (estimated glomerular filtration rate [eGFR] between 60 and <90 mL/min) or moderate (eGFR between 30 and <60 mL/min) renal impairment. No data are currently available in patients with severe renal impairment or on dialysis and no dose recommendations can be given.
Hepatic impairment
The use of FABHALTA® is not recommended in patients with severe hepatic impairment (Child-Pugh class C). No dose adjustment is required for patients with mild (Child-Pugh class A) or moderate (Child-Pugh class B) hepatic impairment.
Paediatric population
The safety and efficacy of FABHALTA® in children aged below 18 years have not been established. No data are available.
Contraindications1
Hypersensitivity to the active substance or to any of the excipients listed below:
Gelatin
Red iron oxide (E172)
Titanium dioxide (E171)
Yellow iron oxide (E172)
Black iron oxide (E172)
Concentrated ammonia solution (E527)
Potassium hydroxide (E525)
Propylene glycol (E1520)
Shellac (E904)Patients who are not currently vaccinated against Neisseria meningitidis and Streptococcus pneumoniae, unless the risk of delaying treatment outweighs the risk of developing an infection from these encapsulated bacteria
Patients with unresolved infection caused by encapsulated bacteria, including Neisseria meningitidis, Streptococcus pneumoniae or Haemophilus influenzae type B, at treatment initiation
Special warnings and precautions1
Serious infections caused by encapsulated bacteria
The use of complement inhibitors, such as FABHALTA®, may predispose individuals to serious, life-threatening or fatal infections caused by encapsulated bacteria. To reduce the risk of infection, all patients must be vaccinated against encapsulated bacteria, including Neisseria meningitidis and Streptococcus pneumoniae. It is recommended to vaccinate patients against Haemophilus influenzae type B if vaccine is available. Healthcare professionals should refer to local vaccination guideline recommendations.
Vaccines should be administered at least 2 weeks prior to administration of the first dose of FABHALTA®. If treatment must be initiated prior to vaccination, patients should be vaccinated as soon as possible and provided with antibacterial prophylaxis until 2 weeks after vaccine administration.
If necessary, patients may be revaccinated in accordance with local vaccination guideline recommendations.
Vaccination reduces, but does not eliminate, the risk of serious infection. Serious infection may rapidly become life-threatening or fatal if not recognised and treated early. Patients should be informed of and monitored for early signs and symptoms of serious infection. Patients should be immediately evaluated and treated if infection is suspected. The use of FABHALTA® during treatment of serious infection may be considered following an assessment of the risks and benefits.
PNH laboratory monitoring
Patients with PNH receiving FABHALTA® should be monitored regularly for signs and symptoms of haemolysis, including measuring lactate dehydrogenase (LDH) levels.
Monitoring of PNH manifestations after treatment discontinuation
If treatment must be discontinued, patients should be closely monitored for signs and symptoms of haemolysis for at least 2 weeks after the last dose. These signs and symptoms include, but are not limited to, elevated LDH levels along with sudden decrease in haemoglobin or PNH clone size, fatigue, haemoglobinuria, abdominal pain, dyspnoea, dysphagia, erectile dysfunction, or major adverse vascular events (MAVEs), including venous or arterial thrombosis. If treatment discontinuation is necessary, alternative therapy should be considered.
If haemolysis occurs after discontinuation of FABHALTA®, restarting treatment should be considered.
Co-administration with other medicinal products
Concomitant use of FABHALTA® with strong inducers of CYP2C8, UGT1A1, PgP, BCRP and OATP1B1/3 has not been studied clinically; therefore, concomitant use is not recommended, due to the potential for reduced efficacy of FABHALTA®. If an alternative concomitant medicinal product cannot be identified, patients should be monitored for potential signs and symptoms of haemolysis.
Educational materials
All physicians who intend to prescribe FABHALTA® must ensure they have received and are familiar with the physician educational materials. Physicians must explain and discuss the benefits and risks of FABHALTA® therapy with the patient and provide them with the patient information pack. The patient should be instructed to seek prompt medical care if they experience any sign or symptom of serious infection or serious haemolysis following treatment discontinuation.
Interaction with other medicinal products and other forms of interaction1
Effects of other medicinal products on FABHALTA®
Strong inducers of CYP2C8, UGT1A1, PgP, BCRP and OATP1B1/3
Although concomitant administration of FABHALTA® with strong inducers of CYP2C8, UGT1A1, PgP, BCRP and OATP1B1/3, such as rifampicin, has not been studied clinically, concomitant use with FABHALTA® is not recommended due to the potential for reduced efficacy of FABHALTA®.
Effects of FABHALTA® on other medicinal products
CYP3A4 substrates
In vitro data showed FABHALTA® has potential for induction of CYP3A4 and may decrease the exposure of sensitive CYP3A4 substrates. The concomitant use of FABHALTA® and sensitive CYP3A4 substrates has not been studied clinically. Caution should be exercised if co-administration of FABHALTA® with sensitive CYP3A4 substrates is required, especially for those with a narrow therapeutic index (e.g. carbamazepine, ciclosporin, ergotamine, fentanyl, pimozide, quinidine, sirolimus, tacrolimus).
CYP2C8 substrates
In vitro data showed FABHALTA® has potential for time-dependent inhibition of CYP2C8 and may increase the exposure of sensitive CYP2C8 substrates, such as repaglinide, dasabuvir or paclitaxel. The concomitant use of FABHALTA® and sensitive CYP2C8 substrates has not been studied clinically. Caution should be exercised if co-administration of FABHALTA® with sensitive CYP2C8 substrates is required.
Fertility, pregnancy and lactation1
Pregnancy
There are no or limited amount of data from the use of FABHALTA® in pregnant women.
PNH in pregnancy is associated with adverse maternal outcomes, including worsening cytopenias, thrombotic events, infections, bleeding, miscarriages and increased maternal mortality, as well as adverse foetal outcomes, including foetal death and premature delivery.
The use of FABHALTA® in pregnant women or women planning to become pregnant may only be considered following a careful assessment of the risk and benefits, if necessary.
Breastfeeding
It is unknown whether FABHALTA® is excreted in human milk. There are no data on the effects of FABHALTA® on the breastfed newborn/infant or on milk production.
A risk to the newborn/infant cannot be excluded. A decision must be made whether to discontinue breastfeeding or to discontinue/abstain from FABHALTA® therapy taking into account the benefit of breastfeeding for the child and the benefit of therapy for the woman.
Fertility
There are no data on the effect of FABHALTA® on human fertility. Available non-clinical data do not suggest an effect of FABHALTA® treatment on fertility.
C5i, C5 inhibitor; EVH, extravascular haemolysis; Hb, haemoglobin; IVH, intravascular haemolysis; LDH, lactate dehydrogenase; MAC, membrane attach complex; PNH, paroxysmal nocturnal haemoglobinuria; QoL, quality of life; RBC, red blood cell.
*FABHALTA® may achieve comprehensive targeting of haemolysis control by controlling terminal complement-mediated IVH and C3-mediated EVH in anti-C5-treated PNH patients and controlling IVH without causing EVH in complement inhibitor-naïve PNH patients.1,11
References
FABHALTA® Summary of Product Characteristics.
Risitano AM, et al. Lancet Haematol 2021;8:e344–e354.
Peffault de Latour R, et al. N Engl J Med 2024;390(11):994-1008.
Gavriilaki E, et al. Blood 2022;139:3571–3582.
Risitano AM, et al. Front Immunol 2019;10:1157.
Shammo J, et al. Hemasphere 2023;7:e22585a2.
Dingli D, et al. Ann Hematol 2022;101:251–263.
Fishman J, et al. Hematol Rep 2023;15:266–282.
Bektas M, et al. J Manag Care Spec Pharm 2020;26:S8–S14.
Brodsky RA. Blood 2014;124:2804–2811.
Schubart A, et al. Proc Natl Acad Sci USA 2019;116:7926–7931.
Janeway CA Jr, et al. Immunobiology: The Immune System in Health and Disease. 5th ed. New York: Garland Science; 2001.
UK | February 2025 | FA-11208255-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.

