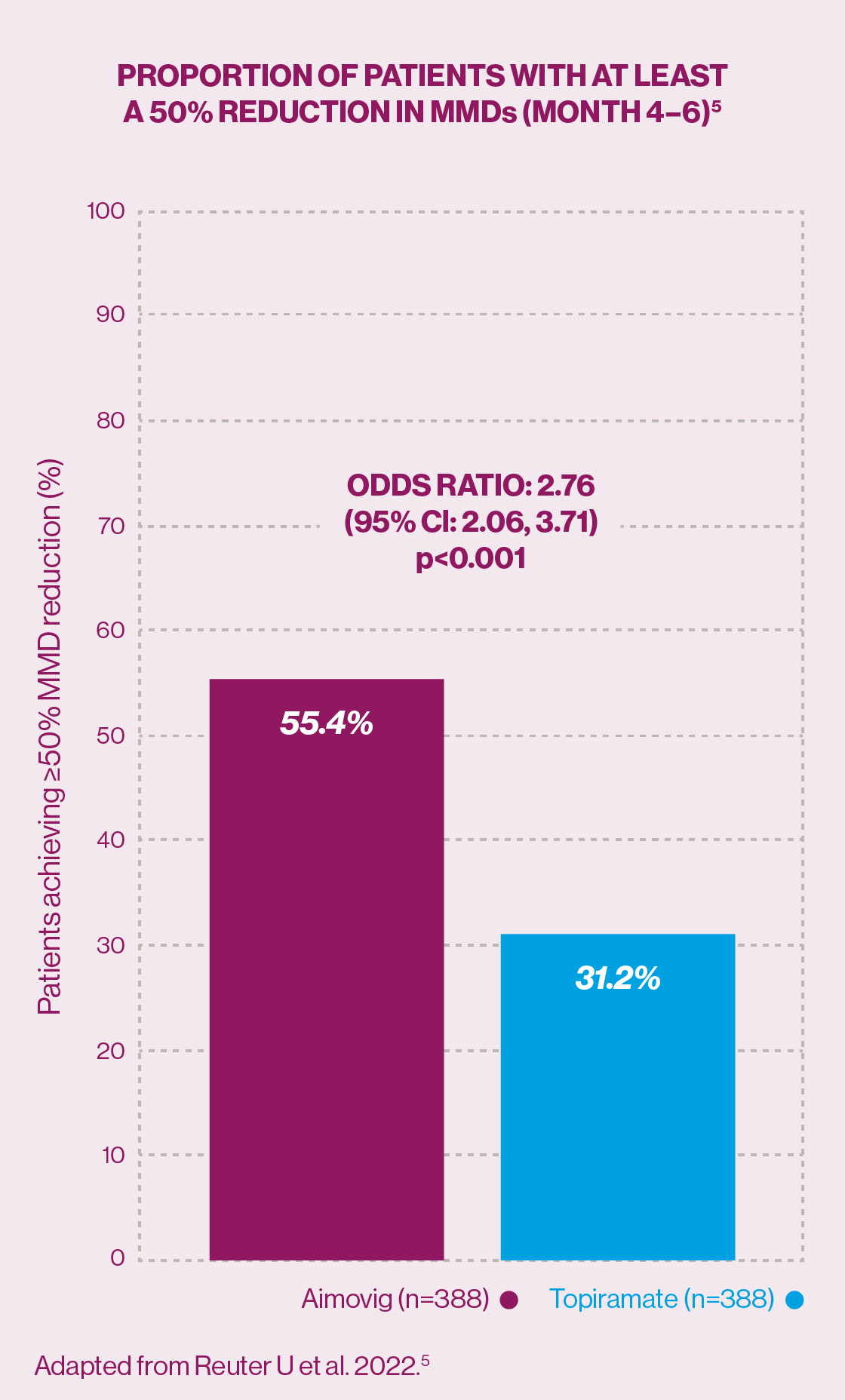Contact us
Contact us to request a Aimovig demonstration kit for training purposes
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
Aimovig is indicated for prophylaxis of migraine in adults who have at least 4 migraine days per month.1
Erenumab is recommended as an option for preventing migraine in adults, only if:
they have 4 or more migraine days a month
at least 3 preventive drug treatments have failed
the 140 mg dose of erenumab is used and
the company provides it according to the commercial arrangement
Stop erenumab after 12 weeks of treatment if:
in episodic migraine (less than 15 headache days a month) the frequency does not reduce by at least 50%
in chronic migraine (15 headache days a month or more with at least 8 of those having features of migraine) the frequency does not reduce by at least 30%
According to the SmPC, the recommended dose is 70 mg erenumab every 4 weeks. Some patients may benefit from a dose of 140 mg every 4 weeks.1
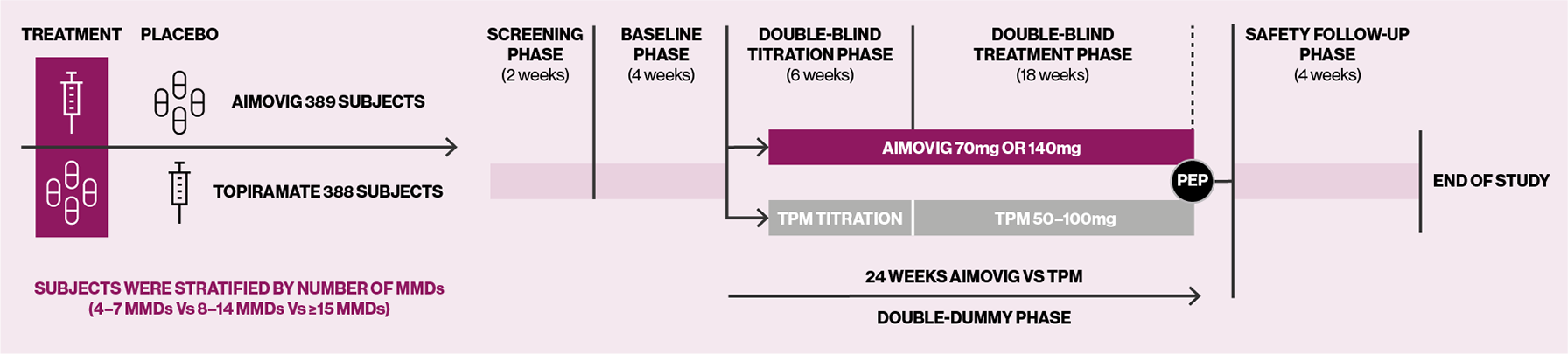
HER-MES was a 24-week, randomised, double-blind, double-dummy, controlled trial conducted in 82 sites in Germany (n=777).5
Secondary endpoint: Significantly more patients achieved a ≥50% reduction in monthly migraine days from baseline with erenumab vs topiramate (55.4% vs 31.2%: odds ratio 2.76; 95% confidence interval 2.06, 3.71; p<0.001 [monthly migraine days at baseline were 10.3 in the erenumab group vs 10.5 in the topiramate group]).5
Aimovig is the first anti-calcitonin gene-related peptide (CGRP) with demonstrated efficacy in ≥50% reduction in monthly migraine days from baseline in a head-to-head trial with SoC topiramate.5
The efficacy and safety profile of Aimovig was evaluated in a 5-year, open-label study, which followed a preceding 12-week double-blind treatment period in patients with episodic migraine.6
The mean (standard error, SE) change in MMDs from a baseline of 8.7 (0.2) days was -5.3 (0.3) days; an average reduction of 62.3% at Year 5.6
The proportions of patients with ≥50%/≥75%/100% reduction in MMDs were maintained throughout the 5-year open-label treatment period with response rates of 71.0%/47.1%/35.5%, respectively, over the last 4-week period.6
Among patients using AMSM at baseline, mean (SE) baseline usage was 6.2 (0.2) treatment days. Mean change from baseline with erenumab was -4.4 (0.3) days over the last 4-week period at Week 268.6
*German trial, no UK patients took part in this study.
AMSM, acute migraine-specific medication; CGRP, calcitonin gene-related peptide; CI, confidence interval; MMD, monthly migraine days; NICE, National Institute of Health and Care Excellence; PEP, primary end point; SE, standard error; SmPC, Summary of Product Characteristics; SoC, standard of care; TPM, topiramate.
References
Aimovig® (erenumab) Summary of Product Characteristics.
Goadsby PJ, et al. Physiol Rev 2017;97(2):553–622.
Hubig LT, et al. Headache 2022;62(9):1187–1197.
National Institute for Health and Care Excellence. Erenumab for preventing migraine. Available at: https://www.nice.org.uk/guidance/ta682/chapter/1-Recommendations [Accessed January 2025].
Reuter U, et al. Cephalalgia 2022;42(2):108–118.
Ashina M, et al. Eur J Neurol 2021;28(5):1716–1725.
UK | January 2025 | FA-11330951
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.