Contact us
Contact us to request a Aimovig demonstration kit for training purposes
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
Aimovig is indicated for prophylaxis of migraine in adults who have at least 4 migraine days per month.1
Initial 12-week treatment phase3
Primary endpoint: Change in monthly migraine days (MMDs).
Baseline MMDs: Aimovig 70 mg group (n=107): 8.6 (SD: 2.5); placebo group (n=160): 8.8 (SD: 2.7).
At Week 12, Aimovig suggests a significant reduction from baseline in MMDs vs placebo (–3.4 days vs –2.3 days respectively; difference –1.1 days [95% CI: –2.1 to –0.2], p=0.021). Further investigation is required from a larger phase III trial.
Adapted from Ashina M, et al. 2021.2
There was no significant difference between Aimovig 70 mg and placebo during the double-blind treatment phase because the study was not designed to detect a significant difference for these endpoints.3
Further investigation from larger Phase III trials is required.2
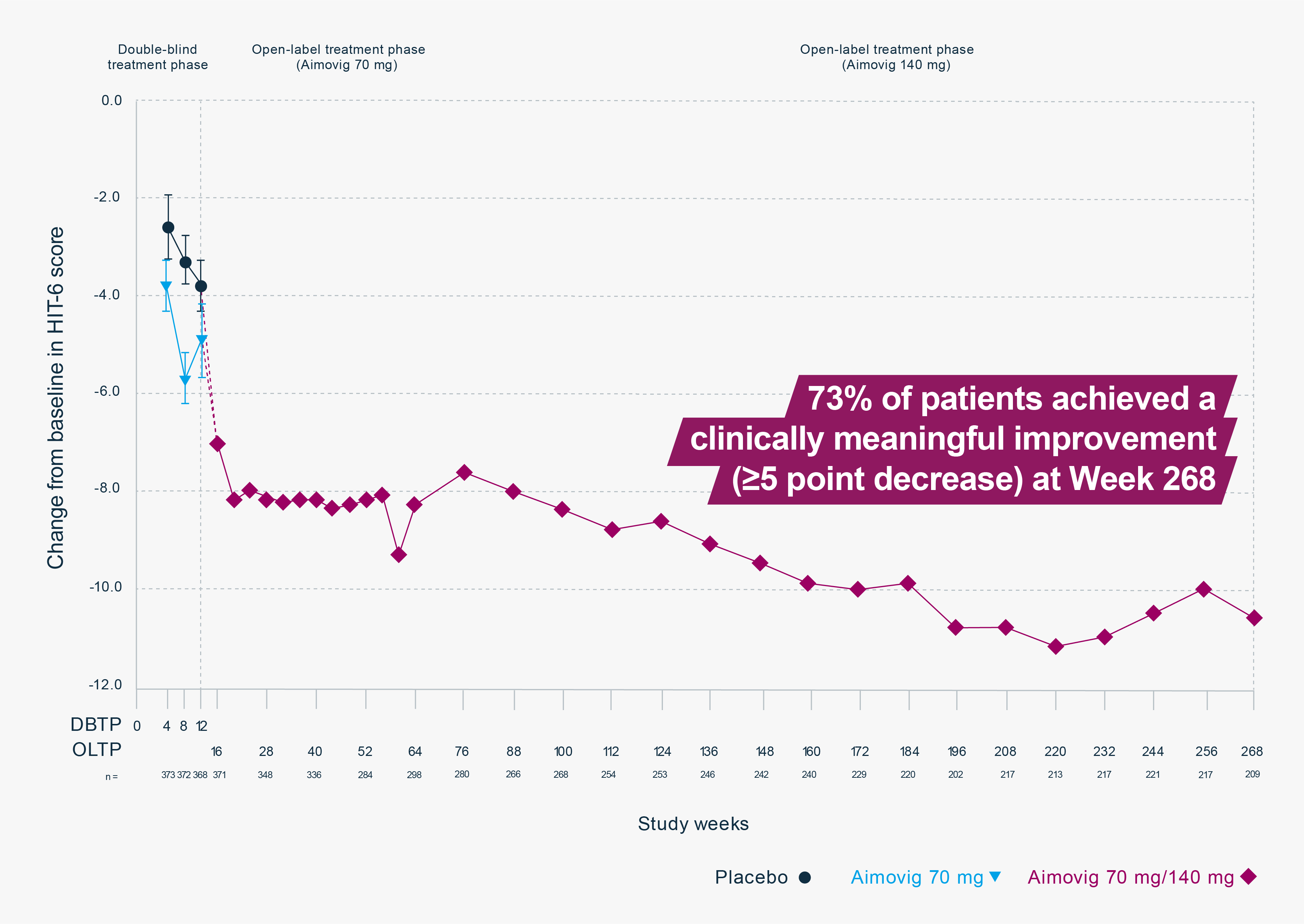
CHU, clinical home use; DBTP, double-blind treatment phase; HIT-6, headache impact test; HRQoL, health-related quality of life; MMD, monthly migraine day; OLTP, open-label treatment phase; PRO, patient-reported outcome; SC, subcutaneously; SD, standard deviation.
This study was a multicentre, open-label 5-year treatment phase, following a 12-week, double-blind, placebo-controlled trial in patients with episodic migraine. In the double-blind treatment phase (12 weeks) patients (n=383) received placebo or Aimovig (7 mg, 21 mg, or 70 mg) SC every 4 weeks. The primary endpoints were the change in monthly migraine days from baseline to Week 12 and number of participants who self-administered a full dose, partial dose, or no dose of erenumab (CHU sub-study). In the open-label treatment phase patients (n=250) received Aimovig every 4 weeks. After exposure to 70 mg, patients increased dose to 140 mg after a protocol amendment. Efficacy endpoints included change in monthly migraine days, change in monthly acute migraine-specific medication days in patients with baseline use and change in HRQoL measured by PROs.2,4
*HIT-6 is a 6-item survey that assesses the adverse impact of headaches on social, role and cognitive functioning, vitality, and psychological distress, providing a summary score.5
References
Aimovig® (erenumab) Summary of Product Characteristics.
Ashina M, et al. Eur J Neurol 2021;28:1716–1725.
Sun H, et al. Lancet Neurol 2016;15(4):382–390.
NHI Clinical Trials. Study to Evaluate the Efficacy and Safety of Erenumab (AMG 334) in Migraine Prevention. Available at: https://www.clinicaltrials.gov/study/NCT01952574 [Accessed December 2024].
Yang M, et al. Cephalalgia 2011;31:357–367.
UK | January 2025 | FA-11330939
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.