Contact us
Any questions? Please click here to contact a member of the Novartis team.
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
Explore our range of useful resources below, designed to support you with your decision making.
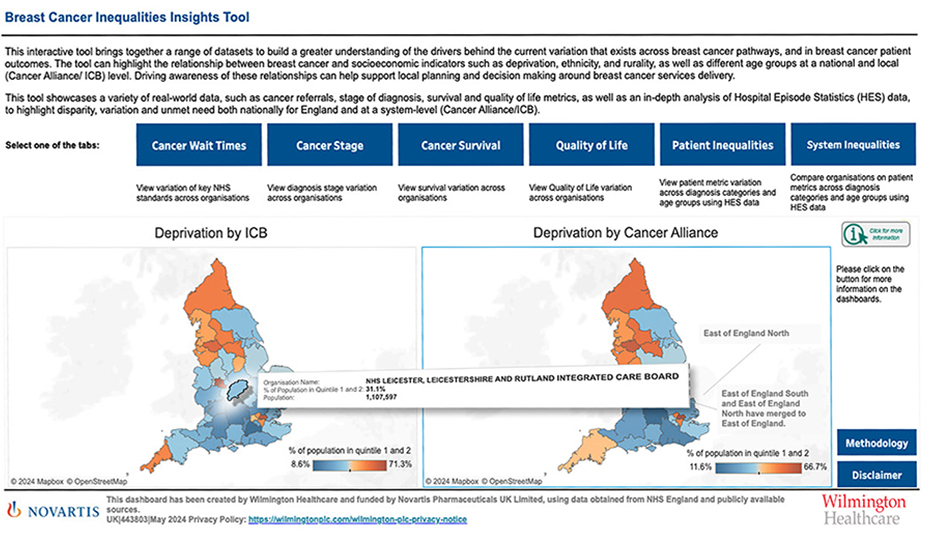
This tool has been created by Wilmington Healthcare and funded by Novartis Pharmaceuticals UK Limited, using data obtained from NHS England and publicly available sources.
This report has been funded by a sponsorship from Novartis Pharmaceuticals UK Limited. Novartis has had no input into the development of the report content or involvement in the delivery or execution of the report.
UK | February 2025 | FA-11222250
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.