Aimovig® (erenumab) efficacy
Aimovig is indicated for prophylaxis of migraine in adults who have at least 4 migraine days per month.1
Reductions in the daily impact of headache may last over 5 years with Aimovig2
Phase II study in patients with episodic migraine2,3
Initial 12-week treatment phase3
Primary endpoint: Change in monthly migraine days (MMDs).
Baseline MMDs: Aimovig 70 mg group (n=107): 8.6 (SD: 2.5); placebo group (n=160): 8.8 (SD: 2.7).
At Week 12, Aimovig suggests a significant reduction from baseline in MMDs vs placebo (–3.4 days vs –2.3 days respectively; difference –1.1 days [95% CI: –2.1 to –0.2], p=0.021). Further investigation is required from a larger phase III trial.
5-year treatment phase: Improvement in HIT-6 score (efficacy endpoint)2
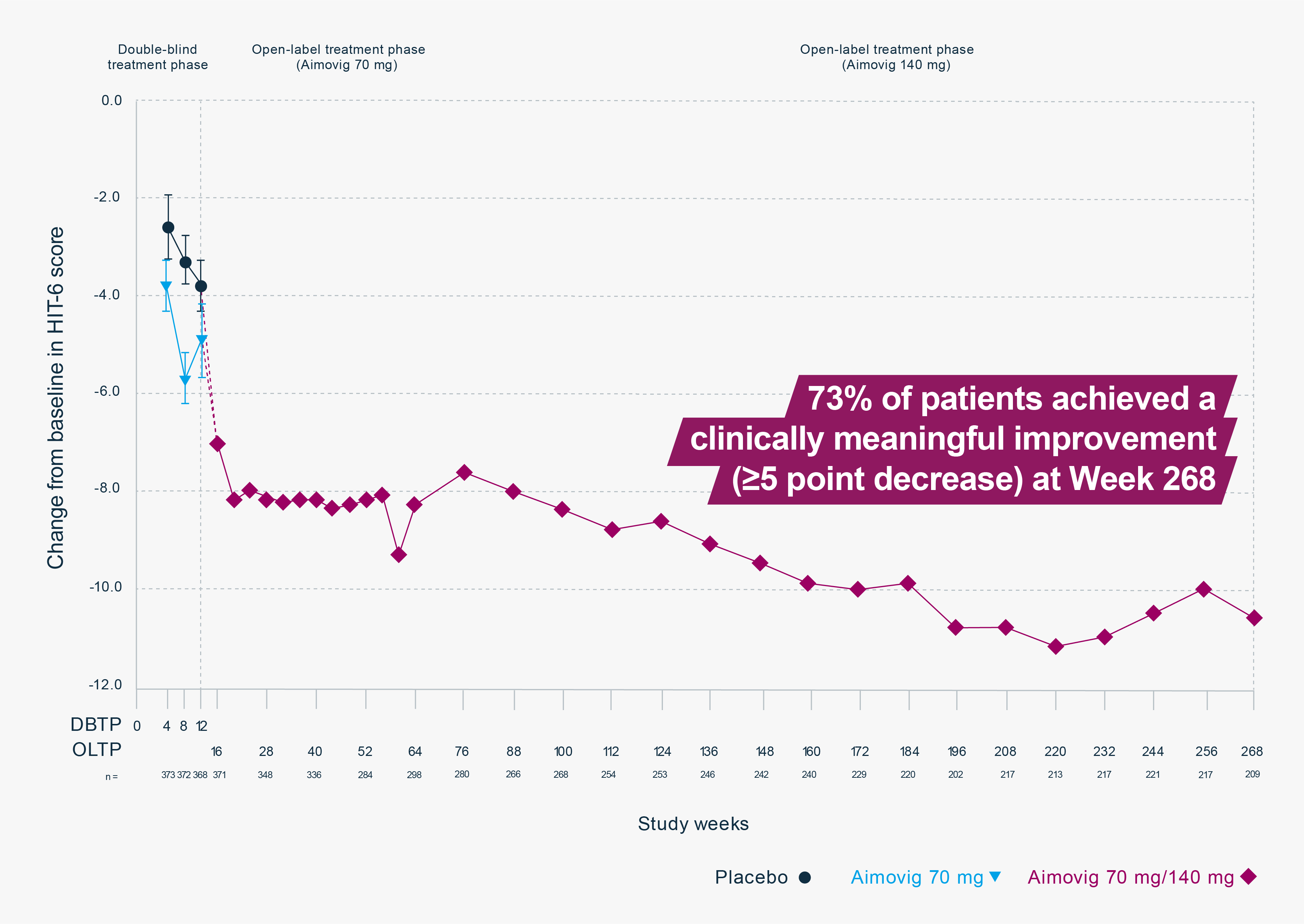
Adapted from Ashina M, et al. 2021.2
There was no significant difference between Aimovig 70 mg and placebo during the double-blind treatment phase because the study was not designed to detect a significant difference for these endpoints.3
Further investigation from larger Phase III trials is required.2
Aimovig provides first long-term data for a migraine-specific preventive treatment over 5 years2,4
CHU, clinical home use; DBTP, double-blind treatment phase; HIT-6, headache impact test; HRQoL, health-related quality of life; MMD, monthly migraine day; OLTP, open-label treatment phase; PRO, patient-reported outcome; SC, subcutaneously; SD, standard deviation.
This study was a multicentre, open-label 5-year treatment phase, following a 12-week, double-blind, placebo-controlled trial in patients with episodic migraine. In the double-blind treatment phase (12 weeks) patients (n=383) received placebo or Aimovig (7 mg, 21 mg, or 70 mg) SC every 4 weeks. The primary endpoints were the change in monthly migraine days from baseline to Week 12 and number of participants who self-administered a full dose, partial dose, or no dose of erenumab (CHU sub-study). In the open-label treatment phase patients (n=250) received Aimovig every 4 weeks. After exposure to 70 mg, patients increased dose to 140 mg after a protocol amendment. Efficacy endpoints included change in monthly migraine days, change in monthly acute migraine-specific medication days in patients with baseline use and change in HRQoL measured by PROs.2,4
*HIT-6 is a 6-item survey that assesses the adverse impact of headaches on social, role and cognitive functioning, vitality, and psychological distress, providing a summary score.5
References
Aimovig® (erenumab) Summary of Product Characteristics.
Ashina M, et al. Eur J Neurol 2021;28:1716–1725.
Sun H, et al. Lancet Neurol 2016;15(4):382–390.
NHI Clinical Trials. Study to Evaluate the Efficacy and Safety of Erenumab (AMG 334) in Migraine Prevention. Available at: https://www.clinicaltrials.gov/study/NCT01952574 [Accessed December 2024].
Yang M, et al. Cephalalgia 2011;31:357–367.
UK | January 2025 | FA-11330939
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.