

This page is intended for UK healthcare professionals only.
FABHALTA® is indicated as monotherapy in the treatment of adult patients with paroxysmal nocturnal haemoglobinuria (PNH) who have haemolytic anaemia.1
FABHALTA®▼ (iptacopan) efficacy and safety profile
The efficacy and safety of FABHALTA® in adult patients with PNH were evaluated in two multicentre, open-label, 24-week Phase III studies: an active comparator-controlled study (APPLY-PNH) and a single-arm study (APPOINT-PNH).
APPLY-PNH (treatment-experienced)
APPLY-PNH study design
APPLY-PNH was a 24-week multicentre, randomised, open-label Phase III study designed to investigate the efficacy and safety of FABHALTA® in treating patients with PNH who continued to experience anaemia despite anti-C5 antibody treatment.1–3
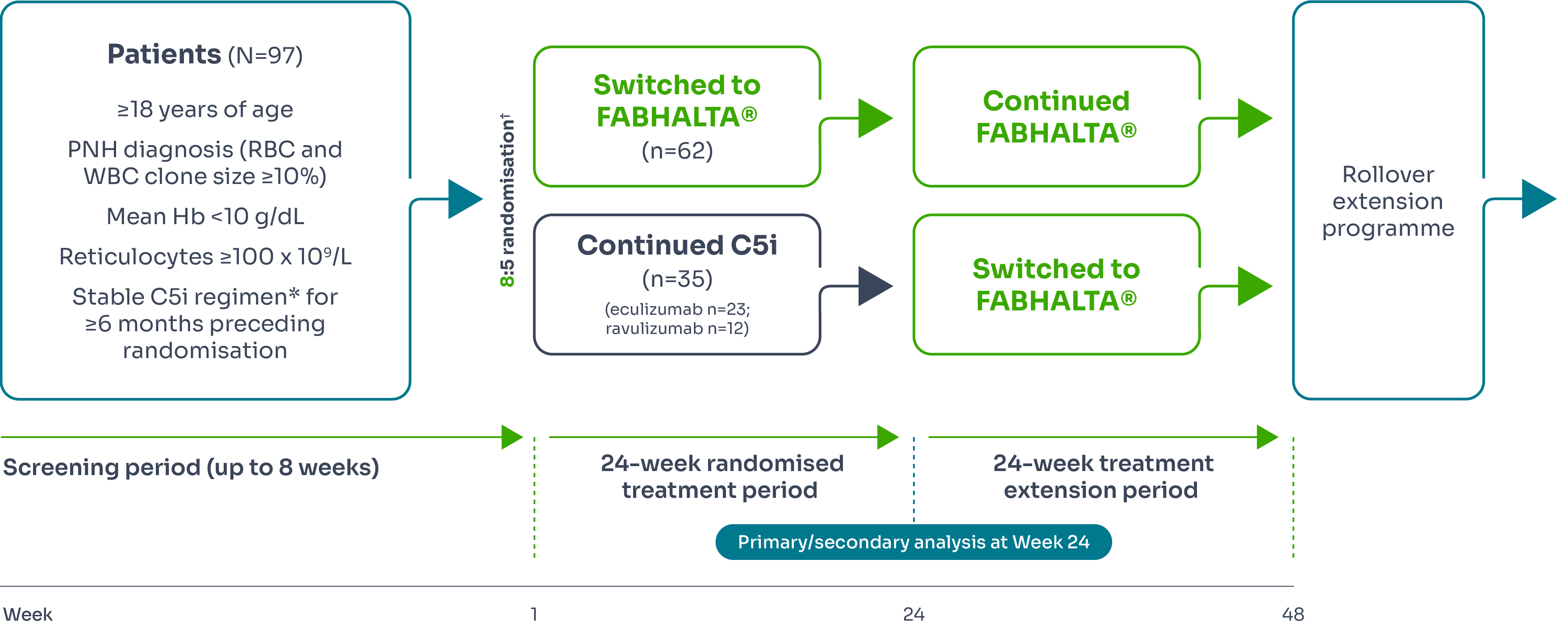
Primary endpoints3 | Secondary endpoints3 |
Efficacy was based on two primary endpoints to demonstrate superiority of FABHALTA® to anti-C5 in achieving haematological response after 24 weeks of treatment, without a need for transfusion, by assessing:
|
|
*Eculizumab or ravulizumab.1,3
†Stratified by prior C5i treatment and RBC transfusions in the preceding 6 months.1,3
‡Assessed between Days 126 and 168.3
§Between Days 14 and 168 and neither meeting the criteria for administration of an RBC transfusion nor receiving an RBC transfusion between Days 14 and 168.3
¶Excluding values within 30 days of RBC transfusion.3
#Throughout the study.3
Hb improvement
Evaluation of efficacy was based on two primary endpoints related to the proportion of participants achieving a haematological response after 24 weeks of treatment (assessed between Days 126 and 168), without a need for RBC transfusion:1
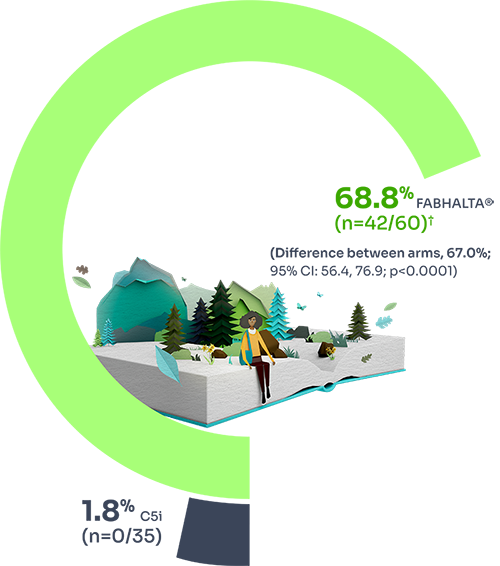
Increase from baseline in Hb level by ≥2 g/dL
Hb normalisation (Hb ≥12 g/dL) rate in the absence of RBC transfusions
82.3% (n=51/60) of patients treated with FABHALTA® achieved sustained increase of ≥2 g/dL in Hb vs 2% (n=0/35) of those treated with C5i (difference between arms = 80.2%; 95% CI: 71.2% to 87.6%; p<0.0001) at Week 24.1
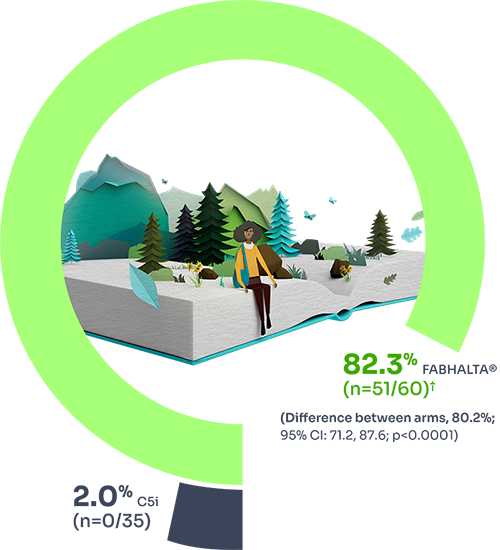
68.8% (n=42/60) of patients treated with FABHALTA® achieved ≥12 g/dL vs 1.8% (n=0/35) of those treated with C5i (difference between arms = 67.0%; 95% CI: 56.4% to 76.9%; p<0.0001) at Week 24.1
*Assessed between Days 126 and 168. Estimated proportions of patients were calculated to reflect the population average probability of a patient meeting the endpoint criteria.3
†Based on observed data among evaluable patients. In 2 patients with partially missing central Hb data between Days 126 and 168, the haematological response could not be established unequivocally. The haematological response was derived using multiple imputation. These patients did not discontinue.1
Transfusion avoidance
Secondary endpoint
Transfusion avoidance may be achieved with FABHALTA®.*1
More patients achieved transfusion avoidance with FABHALTA® vs C5i (94.8% [n=59/62] vs 25.9% [n=14/35]; difference between arms: 68.9%; 95% CI: 51.4 to 83.9; p<0.0001) at Week 24.1
![Graphic showing that more patients achieved transfusion avoidance with FABHALTA® vs c5i (94.8& [n=59/62] vs 25.9% [n=14/35]; difference between arms: 68.9%; 95% Cl: 51.4 to 83.9; p<0.0001) at week 24.¹ Graphic showing that more patients achieved transfusion avoidance with FABHALTA® vs c5i (94.8& [n=59/62] vs 25.9% [n=14/35]; difference between arms: 68.9%; 95% Cl: 51.4 to 83.9; p<0.0001) at week 24.¹](/uk-en/sites/pro_novartis_com_uk/files/2024-10/fabhalta-transfusion-avoidance-graphic-a_2700.png)
37/62 in the FABHALTA® arm and 22/35 in the C5i arm had at least one RBC transfusion in the 12 months prior to trial enrolment.1
*Transfusion independence is defined as absence of administration of packed-RBC transfusions or meeting the criteria for transfusion between Days 14 and 168.1
†Based on observed data among evaluable patients. In 2 patients with partially missing central Hb data between Days 126 and 168, the haematological response could not be established unequivocally. The haematological response was derived using multiple imputation. These patients did not discontinue.1
Hb over time
Secondary endpoint
Change from baseline in Hb levels was a secondary endpoint of the study.1
FABHALTA® demonstrates increases in Hb as early as Week 2, sustained for 24 weeks, vs C5i.1
Mean Hb levels from baseline to Week 24*1
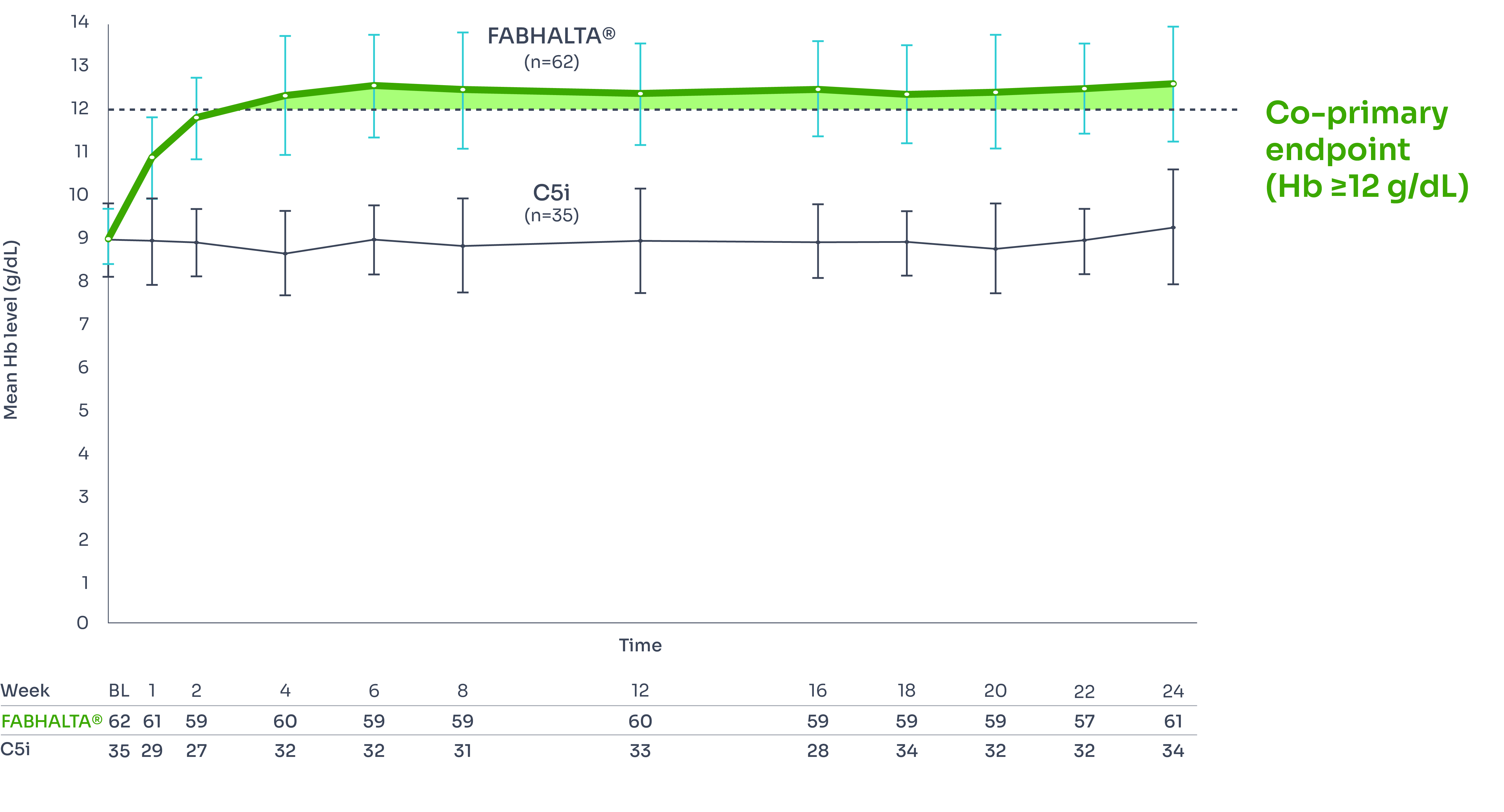
*Includes values within 30 days of RBC transfusion. 2/62 patients in the FABHALTA® arm and 21/35 patients in the C5i arm had RBC transfusions between Days 14 and 168.3
Fatigue
Secondary endpoint
FABHALTA® significantly improves FACIT-Fatigue scores to near-normal vs C5i.3,4
Adjusted mean change from baseline: FABHALTA® +8.59; 95% CI, 6.72–10.47; C5i +0.31; 95% CI, −2.20–2.81.‡3
Difference between arms: +8.29; 95% CI, 5.28–11.29; p<0.0001.‡3
Difference between arms: +8.29; 95% CI, 5.28–11.29; p<0.0001.*3
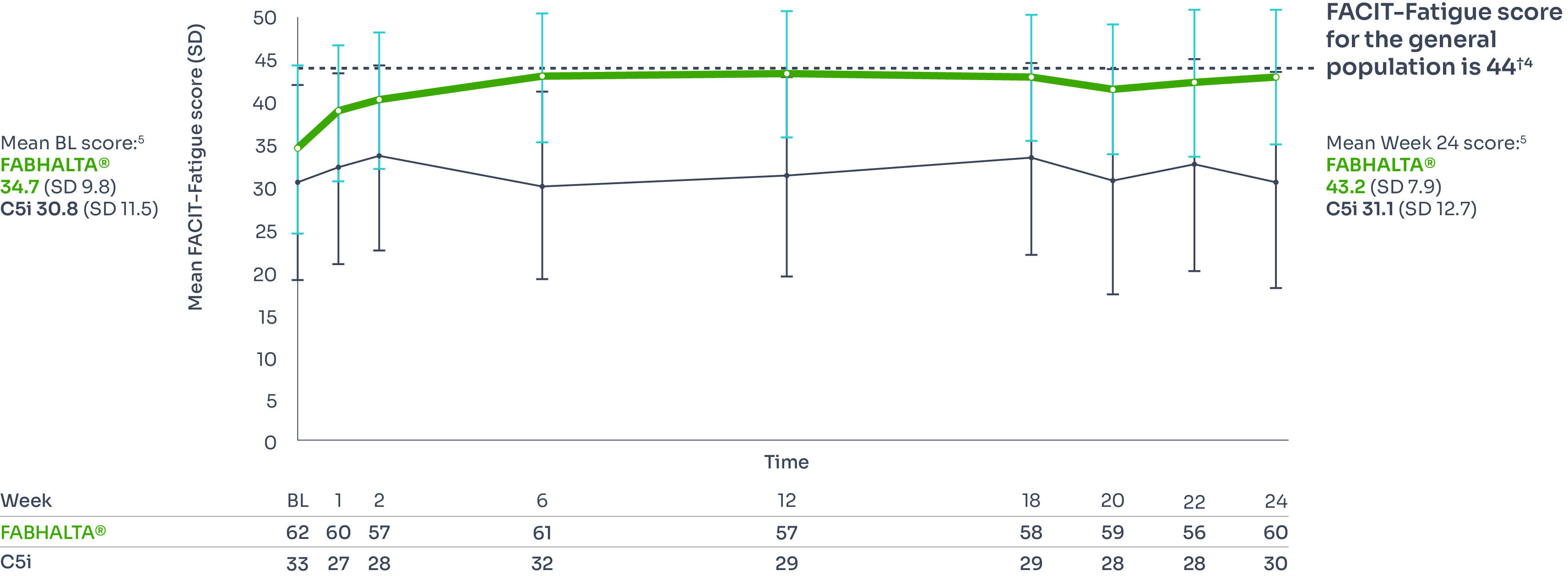
*The Functional Assessment of Chronic Illness Therapy Fatigue (FACIT-F) is a patient-reported measure that assesses fatigue and its impact upon daily activities and function. Scores on the FACIT-Fatigue scale range from 0 to 52, with higher scores indicating less fatigue and 52 indicating no fatigue.4
†Determined through the assessment of 1010 adults in the US in 2002 and 2426 adults in Germany in 2018.4
‡Adjusted mean assessed between Days 126 and 168, values within 30 days after transfusion were included in the analysis.1,3
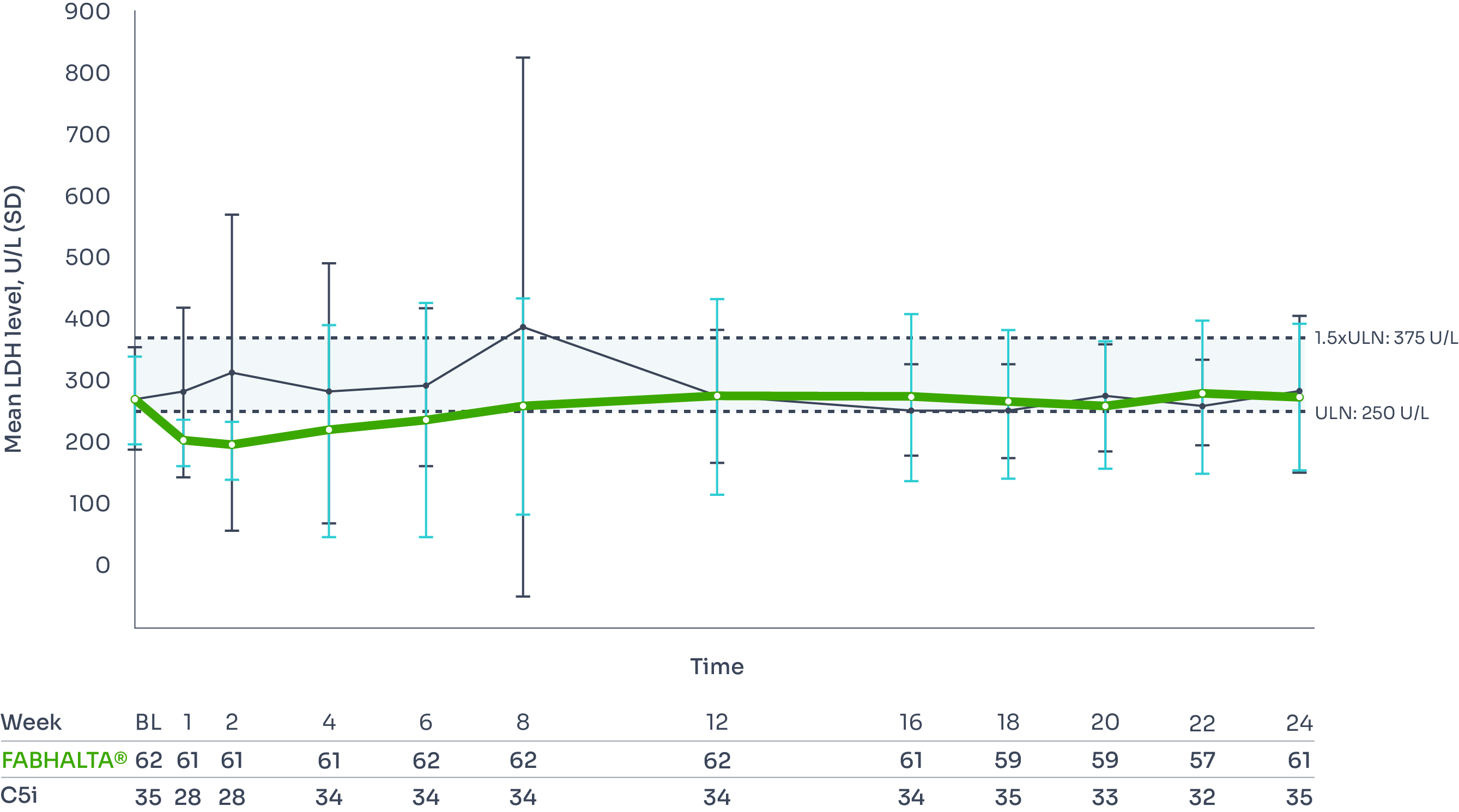
Haemolysis control
Secondary endpoint
LDH ratio to baseline (<1.5 x ULN):1
Adjusted geometric mean ratio to baseline at Week 24*†1
0.96 (n=62) with FABHALTA® vs 0.98 (n=35) with C5i
No statistical difference: (95% CI, −10.18–11.32) with FABHALTA® vs C5i (p=0.84).1,6
Apart from at Week 8, mean LDH level remained <1.5 x ULN in both the FABHALTA® and C5i groups throughout the trial (no significant difference between groups).3
Mean LDH level from baseline to Week 241,3
ARC improvement:
Superior reductions in ARC with FABHALTA® vs C5i1,3
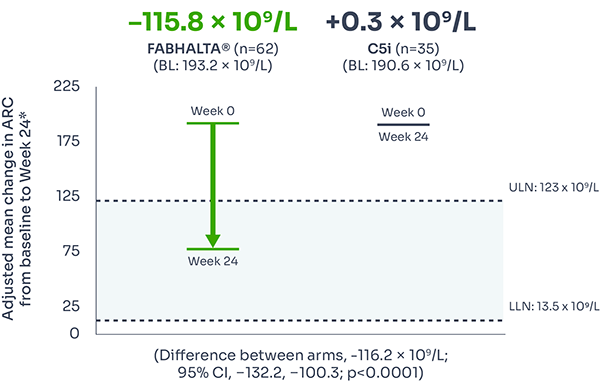
ARC reflects bone marrow function, with levels increased during IVH and EVH.7
Difference between arms, –116.2 x 109/L; 95% CI, –132.2, –100.3; p<0.0001).
ARC reduction was seen as early as Week 2 and was sustained over 24 weeks.3
Mean ARC from baseline to Week 243
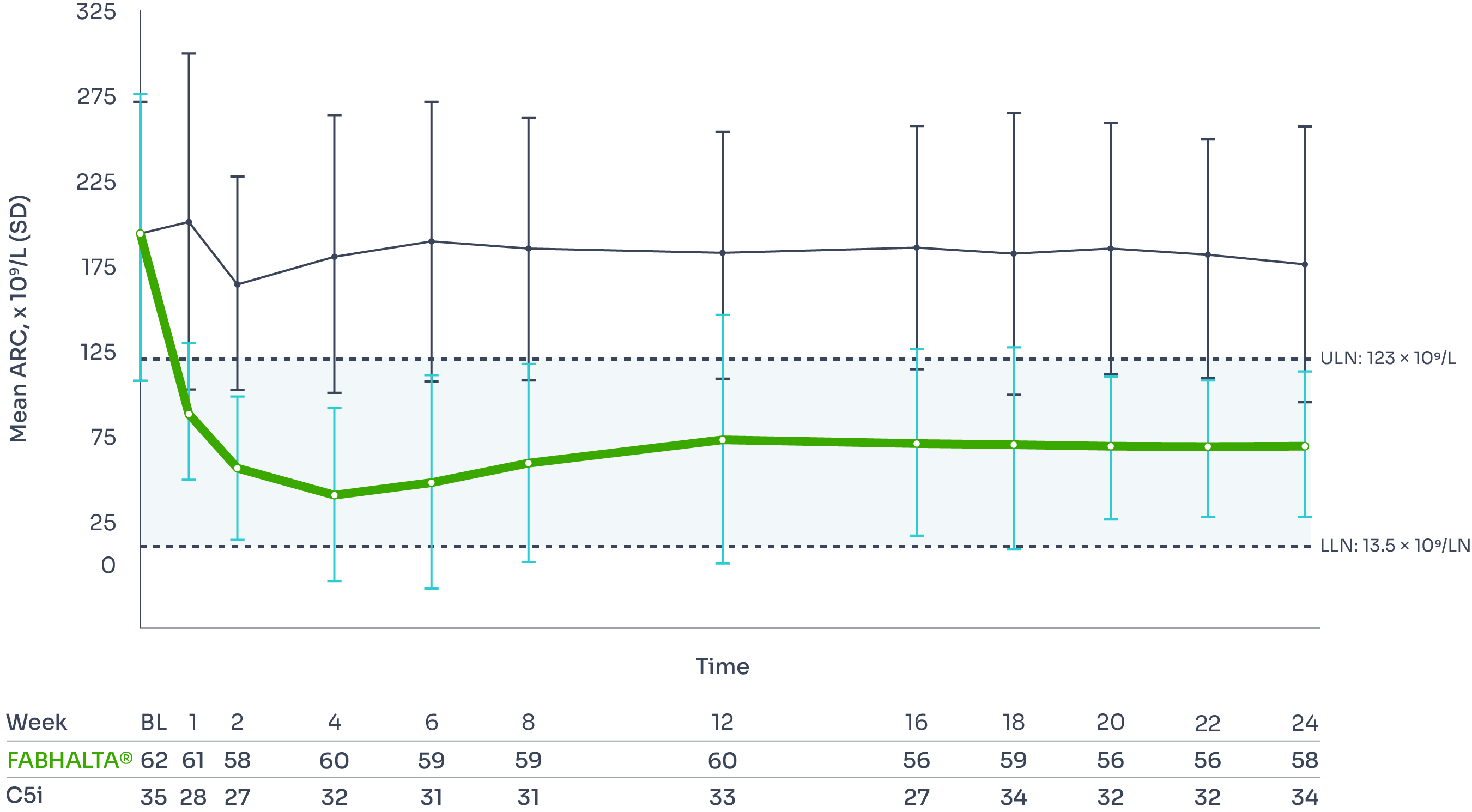
*Assessed between Days 126 and 168, values within 30 days after transfusion were included in the analysis.1
†Difference between arms: ratio=0.99 (95% CI, 0.89–1.10; p=0.84).1
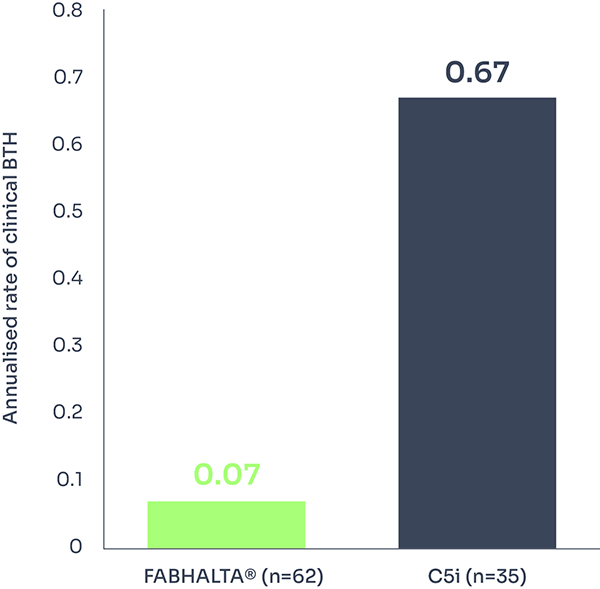
Breakthrough haemolysis
Secondary endpoint
Occurrence of clinical breakthrough haemolysis (BTH)* was a secondary endpoint of the study.3
At Week 24, clinical BTH occurred in 2 out of 62 (3.2%) patients on FABHALTA® and 6 out of 35 (17.1%) patients on C5i.1,3
This represents a 90% reduction in annualised rate of clinical BTH (Rate ratio: 0.10; 95% CI: 0.02 to 0.61; p=0.01).†1
*Clinical BTH was defined as meeting clinical criteria (either decrease of Hb level ≥2 g/dL compared with the last assessment or within 15 days; or signs or symptoms of gross haemoglobinuria, painful crisis, dysphagia, or any other significant clinical PNH-related signs and symptoms) and laboratory criteria (LDH >1.5 x ULN and increased as compared with the last 2 assessments).1
†A negative binomial model was used for the comparison between treatment arms.1
Safety profile
FABHALTA® is generally well tolerated.1,3
Treatment-emergent adverse events in the 24-week core period | FABHALTA® (n=62) N (%) | C5i (n=35) N (%) |
Infections and infestations | ||
Nasopharyngitis | 7 (11) | 2 (6) |
COVID-19 | 5 (8) | 9 (26) |
Urinary tract infection | 5 (8) | 1 (3) |
Nervous system disorders | ||
Headache | 10 (16) | 1 (3) |
Dizziness | 4 (6) | 0 |
Gastrointestinal disorders | ||
Diarrhoea | 9 (15) | 2 (6) |
Abdominal pain | 4 (6) | 1 (3) |
Nausea | 6 (10) | 1 (3) |
Musculoskeletal and connective tissue disorders | ||
Arthralgia | 5 (8) | 1 (3) |
Other | ||
Increased blood LDH | 4 (6) | 3 (9) |
Annualised rate of MAVE | 0.03 (1.6%) | 0 |
Annualised rate of clinical BTH*† | 0.07 (3.2%) | 0.67 (17.1%) |
*Clinical BTH was defined as meeting clinical criteria (either decrease of Hb level ≥2 g/dL compared with the last assessment or within 15 days; or signs or symptoms of gross haemoglobinuria, painful crisis, dysphagia, or any other significant clinical PNH-related signs and symptoms) and laboratory criteria (LDH >1.5 x ULN and increased as compared with the last 2 assessments).1
†During 24 weeks, 2/62 patients experienced clinical BTH with FABHALTA® vs 6/35 with C5i (assessed between Days 1 and 168).3
The most frequently reported adverse events (>10% of patients) on FABHALTA® were headache, diarrhoea and nasopharyngitis3
During the 24 week treatment period, one patient experienced a transient ischaemic attack in the FABHALTA® arm, which was deemed unrelated to treatment by the investigator (assessed between Days 1 and 168)3
Some patients experienced decreases in platelet counts. These were generally mild to moderate although more severe decreases were seen in a few patients with pre-existing thrombocytopenia1
One patient in the FABHALTA® group discontinued treatment due to pregnancy.3 There are no or limited amounts of data from the use of FABHALTA® in pregnant women. The use of FABHALTA® in pregnant women or women planning to become pregnant may only be considered following a careful assessment of the risk and benefits, if necessary1
No patients discontinued FABHALTA® due to adverse events3
No deaths and no serious encapsulated bacteria infections were observed. Please refer to the vaccinations section for additional information prior to prescription3
UTI was a commonly reported SAE1
See the SmPC for full safety information and detailed information on drug–drug interactions.1
BTH, breakthrough haemolysis; C5i, C5 inhibitor; COVID-19, coronavirus disease; Hb, haemoglobin; LDH, lactate dehydrogenase; MAVE, major adverse vascular event; PNH, paroxysmal nocturnal haemoglobinuria; SAE, serious adverse event; SmPC, Summary of Product Characteristics; ULN, upper limit of normal; UTI, urinary tract infection.
APPOINT-PNH (treatment-naïve)
APPOINT-PNH study design
APPOINT-PNH was a 24-week multicentre, single-arm, open-label Phase III study designed to investigate the efficacy and safety of FABHALTA® (iptacopan) in treating patients with PNH who have not received treatment previously with C5 inhibitors.1,3,8
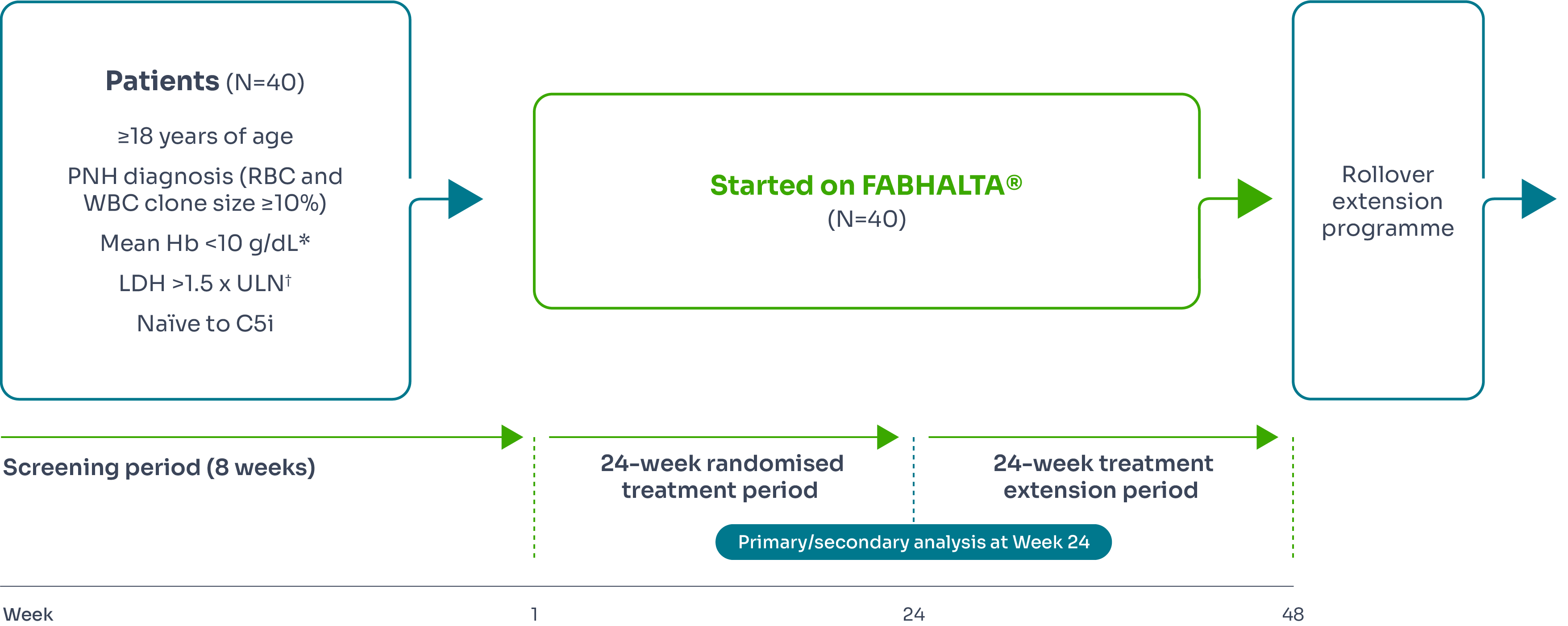
Primary endpoints3 | Secondary endpoints3 |
Haematological response defined as an increase from baseline in Hb of ≥2 g/d‡ in the absence of RBC transfusions§ |
|
*Confirmed by two measurements 2 to 8 weeks apart for patients not receiving an RBC transfusion during screening, or by one measurement during the first screening visit for patients receiving an RBC transfusion.3
†Confirmed by two measurements 2 to 8 weeks apart during screening period.3
‡Assessed between Days 126 and 168.
§Between Days 14 and 168 and neither meeting the criteria for administration of an RBC transfusion nor receiving an RBC transfusion between Days 14 and 168.
¶Excluding values within 30 days of RBC transfusion.
#As per the protocol definition.
||Throughout the study.
Hb improvement
FABHALTA® demonstrated sustained Hb improvements of ≥2 g/dL from baseline (8.2 g/dL) in the absence of transfusions at Week 24 in 92.2% (n=31/33) of patients.1,3,9

The secondary endpoint of Hb improvement ≥12 g/dL from baseline was observed in 62.8% (n=19/33) of patients treated with FABHALTA®.1
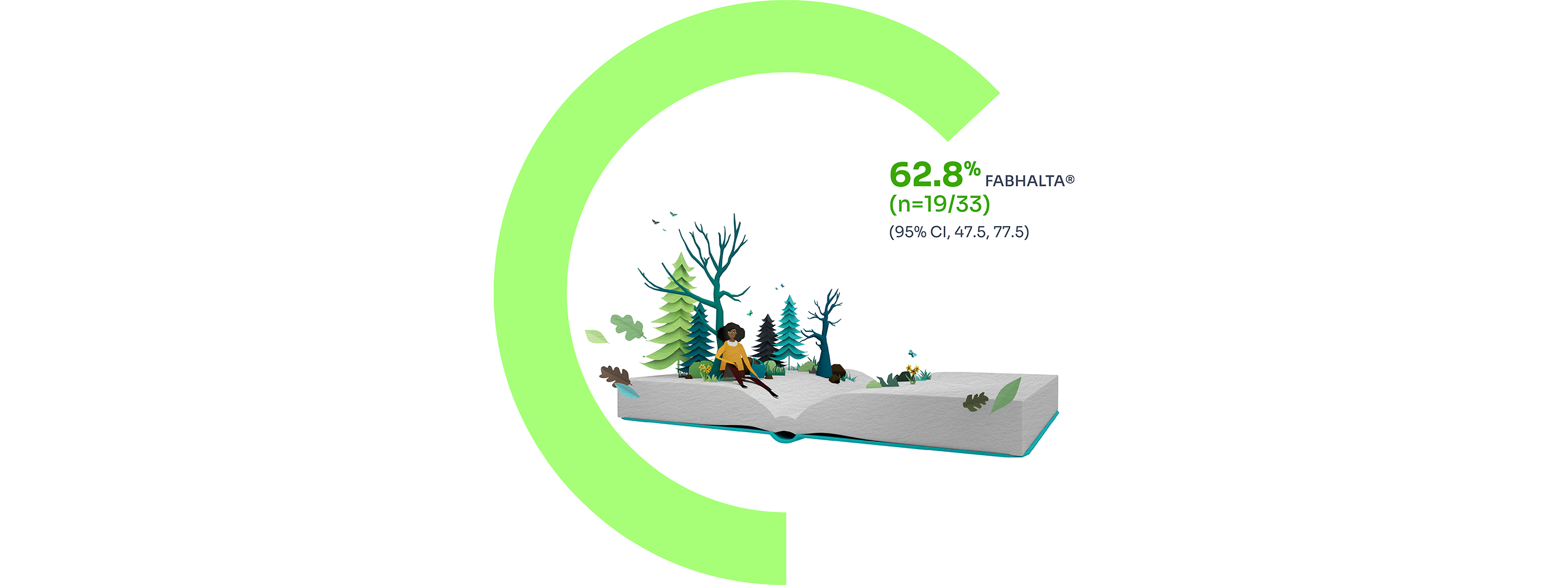
7 patients had partially missing central haemoglobin data between Days 126 and 168. The haematological response was derived using multiple imputation.9
The increase of Hb from baseline was sustained over 24 weeks:1,3
Mean Hb levels from baseline to Week 24*3
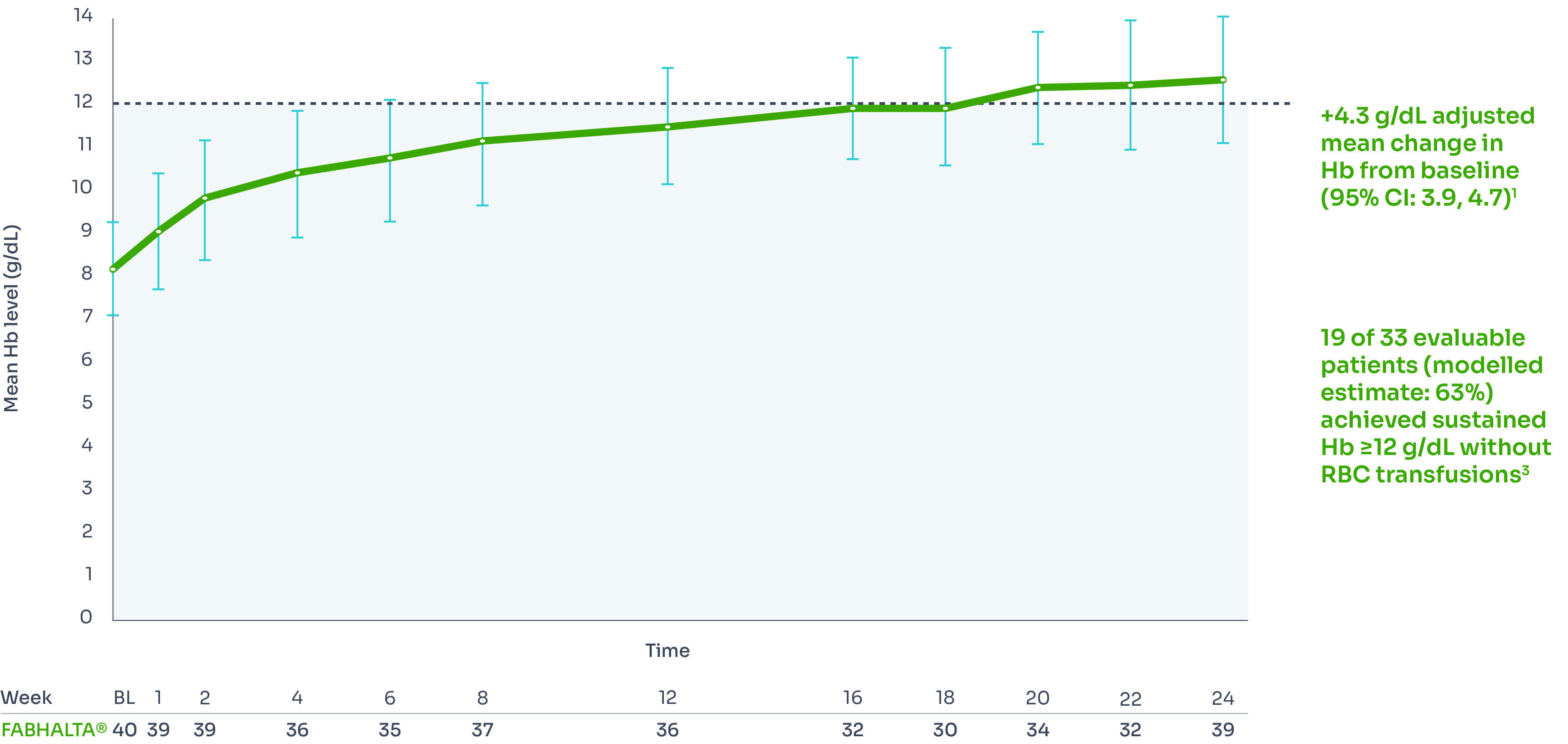
*Assessed between Days 126 and 168. Estimated proportions of patients were calculated to reflect the population average probability of a patient meeting the endpoint criteria.3
†Includes values within 30 days of RBC transfusion. RBC transfusions were received by 5/40 patients between Days 1 and 13 and by 0/40 patients between Days 14 and 168.3
Transfusion avoidance
Secondary endpoint
Transfusion avoidance may be achieved with FABHALTA®.1
97.6% (n=40/40) achieved transfusion avoidance by Week 24 (95% CI, 92.5–100.0).*1

28/40 had at least one RBC transfusion in the 6 months prior to treatment.
*Transfusion avoidance is defined as absence of administration of packed-RBC transfusions or meeting the criteria for transfusion between Days 14 and 168.1
Fatigue
Secondary endpoint
FABHALTA® demonstrated improvements in FACIT-Fatigue levels from baseline.3
Mean FACIT-Fatigue scores over 24 weeks*3

Adjusted mean change from baseline with FABHALTA® +10.75; 95% CI, 8.66–12.84.‡3,10
*The Functional Assessment of Chronic Illness Therapy Fatigue (FACIT-F) is a patient-reported measure that assesses fatigue and its impact upon daily activities and function. Scores on the FACIT-Fatigue scale range from 0 to 52, with higher scores indicating less fatigue and 52 indicating no fatigue.4
†Determined through the assessment of 1010 adults in the US in 2002 and 2426 adults in Germany in 2018.4
‡Between Days 126 and 168.3
Haemolysis control
Secondary endpoint
FABHALTA® may offer haemolysis control by reducing LDH and ARC from baseline.1
LDH (<1.5 x ULN):
95% of patients had LDH ≤1.5 × ULN at Week 24:
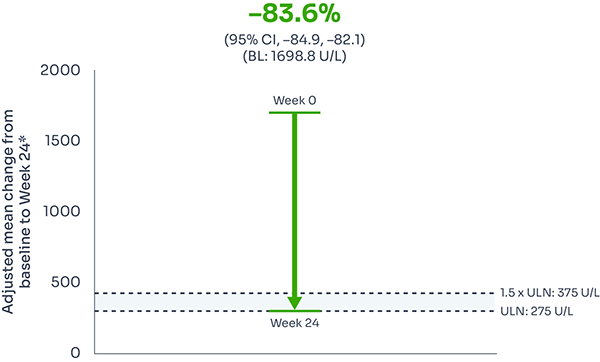
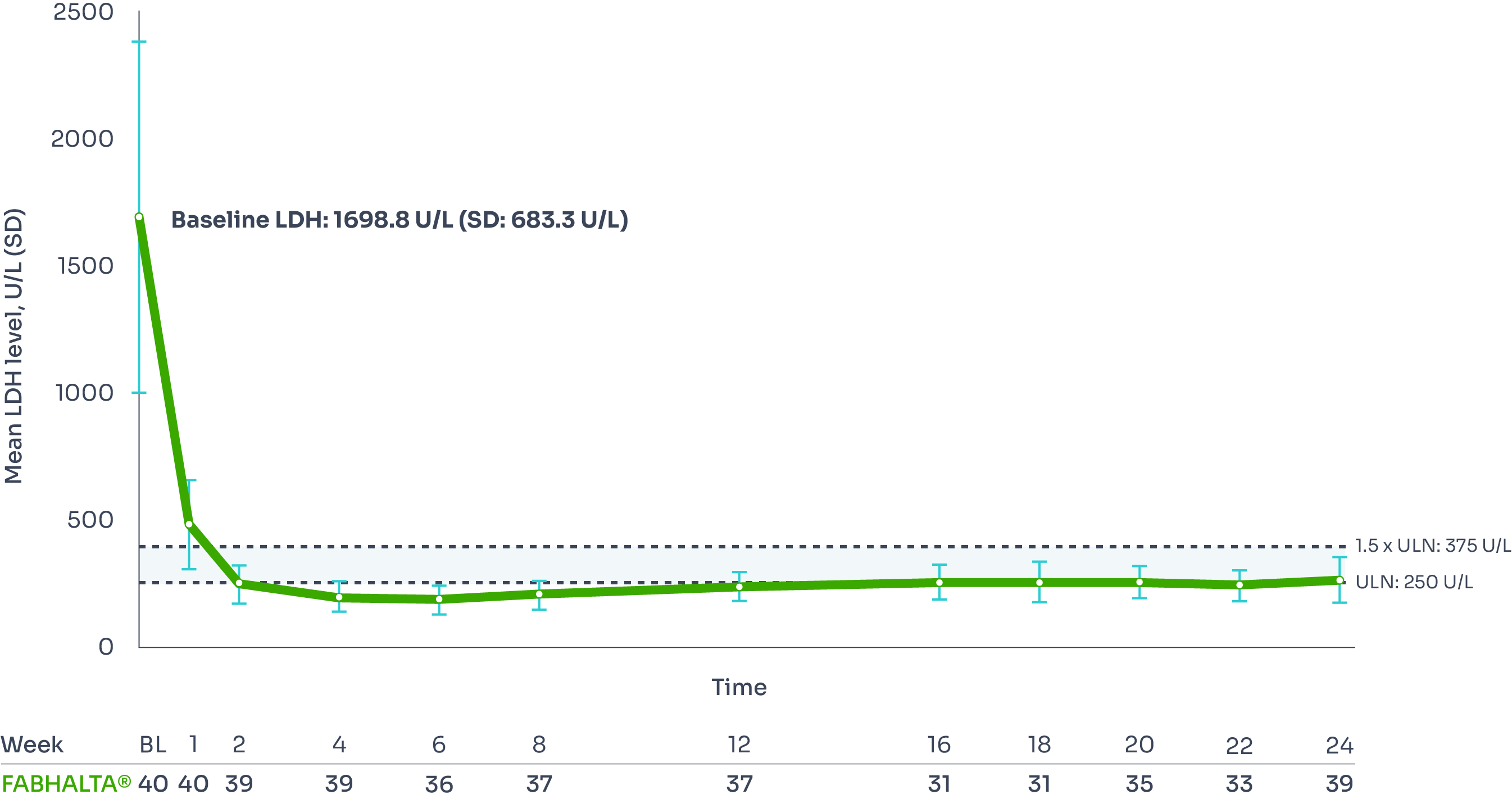
Reductions in LDH were seen as early as Week 1, and were sustained over 24 weeks, with 95% of patients achieving LDH ≤1.5 x ULN at Week 24.1,2
Adjusted mean LDH change from baseline: –83.6% (95% CI: –84.9, –82.1).9
Mean LDH level from baseline to Week 241,3
ARC reduction:
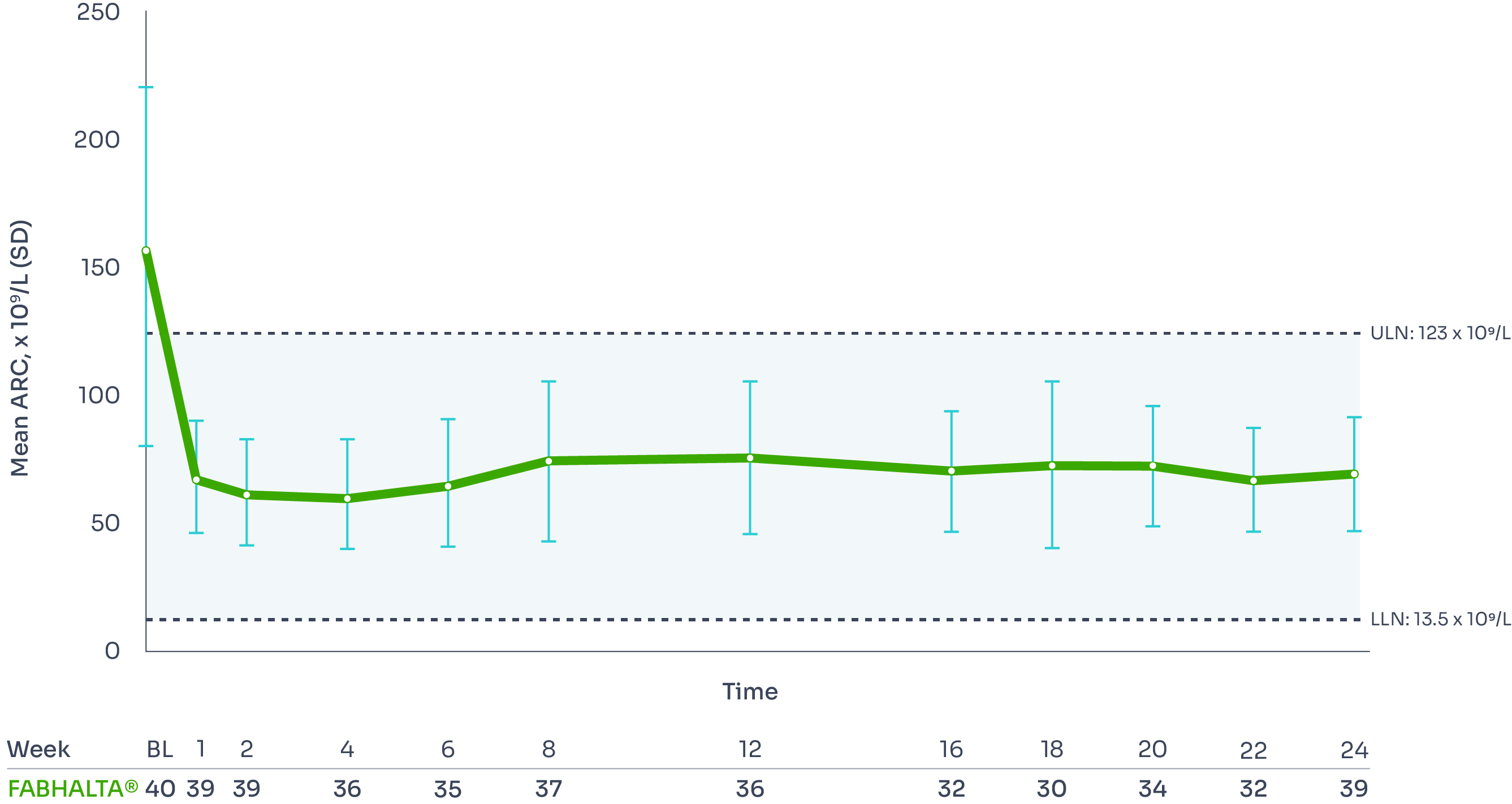
FABHALTA® reduced ARC from baseline by −82.5% at Week 24:1,3
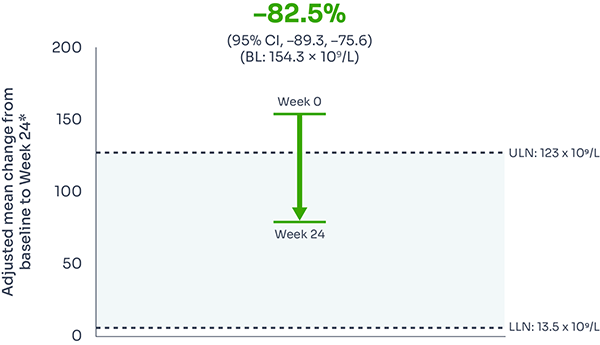
ARC reduction was observed with FABHALTA® as early as Week 1, and was sustained over 24 weeks.3
*Assessed between Days 126 and 168.1
Mean ARC from baseline to Week 241
Safety profile
FABHALTA® is generally well tolerated.*1,3
Treatment-emergent adverse events in the 24-week core period | FABHALTA® (n=62) N (%) |
Infections and infestations | |
Upper respiratory tract infection | 5 (12) |
COVID-19 | 6 (15) |
Blood and lymphatic system disorders | |
Iron deficiency | 3 (8) |
Nervous system disorders | |
Headache | 11 (28) |
Gastrointestinal disorders | |
Diarrhoea | 3 (8) |
Other | |
Annualised rate of MAVE† | 0 |
Annualised rate of clinical BTH†‡ | 0 |
Serious treatment-emergent adverse events | |
Bacterial pneumonia§ | 1 (3) |
Cataract | 1 (3) |
COVID-19 | 1 (3) |
Type II diabetes mellitus | 1 (3) |
†During 24-week core treatment period.3
‡Clinical BTH was defined as meeting clinical criteria (either decrease of Hb level ≥2 g/dL compared with the last assessment or within 15 days; or signs or symptoms of gross haemoglobinuria, painful crisis, dysphagia, or any other significant clinical PNH-related signs and symptoms) and laboratory criteria (LDH >1.5 x ULN and increased as compared with the last 2 assessments).1
§Severe treatment-emergent adverse event of lobar pneumonia of bacterial aetiology, for which no causative organism was identified and which resolved with antibiotic treatment.3
The most frequently reported adverse events (>10% of patients) were upper respiratory tract infections, headache and COVID-193
No deaths were observed3
One patient experienced a serious encapsulated bacteria infection;|| please refer to the FABHALTA® SmPC for additional information prior to prescription3
No patients discontinued due to adverse events3
See the SmPC for full safety information and detailed information on drug–drug interactions.1
BTH, breakthrough haemolysis; COVID-19, coronavirus disease; Hb, haemoglobin; LDH, lactate dehydrogenase; MAVE, major adverse vascular event; PNH, paroxysmal nocturnal haemoglobinuria; SmPC, Summary of Product Characteristics; ULN, upper limit of normal.
*The safety profile of FABHALTA® was established in treatment-naïve patients in the APPOINT-PNH Phase III clinical study; 24-week data shown.3
||Bacterial pneumonia.3
Summary of the safety profile
The most commonly reported adverse reactions are upper respiratory tract infection (18.9%), headache (18.3%) and diarrhoea (11.0%). The most commonly reported serious adverse reaction was urinary tract infection (1.2%).1
Tabulated list of adverse reactions
The table below shows the adverse reactions observed in the clinical studies with FABHALTA® in patients with PNH. Adverse reactions are listed by MedDRA system organ class (SOC) and frequency, using the following convention: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to <1/1,000) or very rare (<1/10,000).1
System organ class adverse reaction | Frequency category |
Infections and infestations | |
Upper respiratory tract infectiona | Very common |
Urinary tract infectionb | Common |
Bronchitisc | Common |
Pneumonia bacterial | Uncommon |
Blood and lymphatic system disorders | |
Platelet count decreased | Common |
Nervous system disorders | |
Headached | Very common |
Dizziness | Common |
Gastrointestinal disorders | |
Diarrhoea | Very common |
Abdominal paine | Common |
Nausea | Common |
Skin and subcutaneous tissue disorders | |
Urticaria | Uncommon |
Musculoskeletal and connective tissue disorders | |
Arthralgia | Common |
a. Upper respiratory tract infection includes preferred terms influenza, nasopharyngitis, pharyngitis, rhinitis, sinusitis, and upper respiratory tract infection. b. Urinary tract infection includes preferred terms urinary tract infection and cystitis escherichia. c. Bronchitis includes preferred terms bronchitis, bronchitis haemophilus and bronchitis bacterial. d. Headache includes preferred terms headache and head discomfort. e. Abdominal pain includes preferred terms abdominal pain, abdominal pain upper, abdominal tenderness and abdominal discomfort. | |
Description of selected adverse reactions1
Platelet count decreased
Decrease in platelet count events was reported in 12/164 (7%) patients with PNH. Of these, 5 patients had events of mild severity, 5 had moderate events and 2 had severe events. Patients with severe events had concurrent anti-platelet antibodies or idiopathic bone marrow aplasia with pre-existing thrombocytopenia. The events started within the first 2 months of iptacopan treatment in 7/12 patients, and after a longer exposure (111 to 951 days) in 5/12 patients. At the cut-off date, 7 (58%) patients had recovered or events were resolving and iptacopan treatment was continued throughout in all patients.
Infections
In PNH clinical studies 1/164 (0.6%) PNH patients reported serious bacterial pneumonia while receiving treatment with iptacopan; the patient had been vaccinated against Neisseria meningitidis, Streptococcus pneumoniae and Haemophilus influenzae type B and recovered following treatment with antibiotics while continuing treatment with iptacopan.
Blood cholesterol and blood pressure increases
In patients treated with iptacopan 200 mg twice a day in PNH clinical studies, mean increases from baseline of approximately 0.7 mmol/l were seen at Month 6 for total cholesterol and LDL-cholesterol. The mean values remained within the normal ranges. Increases in blood pressure, particularly diastolic blood pressure (DBP), were observed (mean increase 4.7 mmHg at Month 6). The mean DBP did not exceed 80 mmHg. Total cholesterol, LDL-C and DBP increases correlated with increases in haemoglobin (improvement in anaemia) in patients with PNH.
Heart rate decrease
In patients treated with iptacopan 200 mg twice a day in PNH clinical studies, a mean decrease in heart rate of approximately 5 bpm was seen at Month 6 (mean of 68 bpm).
Special populations1
Elderly
No dose adjustment is required for patients 65 years of age and older.
Renal impairment
No dose adjustment is required in patients with mild (estimated glomerular filtration rate [eGFR] between 60 and <90 ml/min) or moderate (eGFR between 30 and <60 ml/min) renal impairment. No data are currently available in patients with severe renal impairment or on dialysis and no dose recommendations can be given.
Hepatic impairment
The use of iptacopan is not recommended in patients with severe hepatic impairment (Child-Pugh class C). No dose adjustment is required for patients with mild (Child-Pugh class A) or moderate (Child-Pugh class B) hepatic impairment.
Paediatric population
The safety and efficacy of iptacopan in children aged below 18 years have not been established. No data are available.
Contraindications1
Hypersensitivity to the active substance or to any of the excipients listed below:
Gelatin
Red iron oxide (E172)
Titanium dioxide (E171)
Yellow iron oxide (E172)
Black iron oxide (E172)
Concentrated ammonia solution (E527)
Potassium hydroxide (E525)
Propylene glycol (E1520)
Shellac (E904)
Patients who are not currently vaccinated against Neisseria meningitidis and Streptococcus pneumoniae, unless the risk of delaying treatment outweighs the risk of developing an infection from these encapsulated bacteria
Patients with unresolved infection caused by encapsulated bacteria, including Neisseria meningitidis, Streptococcus pneumoniae or Haemophilus influenzae type B, at treatment initiation
Special warnings and precautions1
Serious infections caused by encapsulated bacteria
The use of complement inhibitors, such as FABHALTA®, may predispose individuals to serious, life-threatening or fatal infections caused by encapsulated bacteria. To reduce the risk of infection, all patients must be vaccinated against encapsulated bacteria, including Neisseria meningitidis and Streptococcus pneumoniae. It is recommended to vaccinate patients against Haemophilus influenzae type B if vaccine is available. Healthcare professionals should refer to local vaccination guideline recommendations.
Vaccines should be administered at least 2 weeks prior to administration of the first dose of FABHALTA®. If treatment must be initiated prior to vaccination, patients should be vaccinated as soon as possible and provided with antibacterial prophylaxis until 2 weeks after vaccine administration.
If necessary, patients may be revaccinated in accordance with local vaccination guideline recommendations.
Vaccination reduces, but does not eliminate, the risk of serious infection. Serious infection may rapidly become life-threatening or fatal if not recognised and treated early. Patients should be informed of and monitored for early signs and symptoms of serious infection. Patients should be immediately evaluated and treated if infection is suspected. The use of FABHALTA® during treatment of serious infection may be considered following an assessment of the risks and benefits.
PNH laboratory monitoring
Patients with PNH receiving FABHALTA® should be monitored regularly for signs and symptoms of haemolysis, including measuring lactate dehydrogenase (LDH) levels.
Monitoring of PNH manifestations after treatment discontinuation
If treatment must be discontinued, patients should be closely monitored for signs and symptoms of haemolysis for at least 2 weeks after the last dose. These signs and symptoms include, but are not limited to, elevated LDH levels along with sudden decrease in haemoglobin or PNH clone size, fatigue, haemoglobinuria, abdominal pain, dyspnoea, dysphagia, erectile dysfunction, or major adverse vascular events (MAVEs), including venous or arterial thrombosis. If treatment discontinuation is necessary, alternative therapy should be considered.
If haemolysis occurs after discontinuation of FABHALTA®, restarting treatment should be considered.
Co-administration with other medicinal products
Concomitant use of FABHALTA® with strong inducers of CYP2C8, UGT1A1, PgP, BCRP and OATP1B1/3 has not been studied clinically; therefore, concomitant use is not recommended, due to the potential for reduced efficacy of FABHALTA®. If an alternative concomitant medicinal product cannot be identified, patients should be monitored for potential signs and symptoms of haemolysis.
Educational materials
All physicians who intend to prescribe FABHALTA® must ensure they have received and are familiar with the physician educational materials. Physicians must explain and discuss the benefits and risks of FABHALTA® therapy with the patient and provide them with the patient information pack. The patient should be instructed to seek prompt medical care if they experience any sign or symptom of serious infection or serious haemolysis following treatment discontinuation.
Interaction with other medicinal products and other forms of interaction1
Effects of other medicinal products on FABHALTA®
Strong inducers of CYP2C8, UGT1A1, PgP, BCRP and OATP1B1/3
Although concomitant administration of FABHALTA® with strong inducers of CYP2C8, UGT1A1, PgP, BCRP and OATP1B1/3, such as rifampicin, has not been studied clinically, concomitant use with FABHALTA® is not recommended due to the potential for reduced efficacy of FABHALTA®.
Effects of FABHALTA® on other medicinal products
CYPЗА4 substrates
In vitro data showed FABHALTA® has potential for induction of CYP3A4 and may decrease the exposure of sensitive CYP3A4 substrates.
The concomitant use of FABHALTA® and sensitive CYP3A4 substrates has not been studied clinically. Caution should be exercised if co-administration of FABHALTA® with sensitive CYP3A4 substrates is required, especially for those with a narrow therapeutic index (e.g. carbamazepine, ciclosporin, ergotamine, fentanyl, pimozide, quinidine, sirolimus, tacrolimus).
CYP2C8 substrates
In vitro data showed FABHALTA® has potential for time-dependent inhibition of CYP2C8 and may increase the exposure of sensitive CYP2C8 substrates, such as repaglinide, dasabuvir or paclitaxel. The concomitant use of FABHALTA® and sensitive CYP2C8 substrates has not been studied clinically. Caution should be exercised if co-administration of FABHALTA® with sensitive CYP2C8 substrates is required.
Fertility, pregnancy and lactation1
Pregnancy
There are no or limited amount of data from the use of FABHALTA® in pregnant women.
PNH in pregnancy is associated with adverse maternal outcomes, including worsening cytopenias, thrombotic events, infections, bleeding, miscarriages and increased maternal mortality, as well as adverse foetal outcomes, including foetal death and premature delivery.
The use of FABHALTA® in pregnant women or women planning to become pregnant may only be considered following a careful assessment of the risk and benefits, if necessary.
Breastfeeding
It is unknown whether FABHALTA® is excreted in human milk. There are no data on the effects of FABHALTA® on the breastfed newborn/infant or on milk production.
A risk to the newborn/infant cannot be excluded. A decision must be made whether to discontinue breastfeeding or to discontinue/abstain from FABHALTA® therapy taking into account the benefit of breastfeeding for the child and the benefit of therapy for the woman.
Fertility
There are no data on the effect of FABHALTA® on human fertility. Available non-clinical data do not suggest an effect of FABHALTA® treatment on fertility.
ARC, absolute reticulocyte count; BL, baseline; BTH, breakthrough haemolysis; C5i, C5 inhibitor; CI, confidence interval; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; EVH, extravascular haemolysis; FACIT, Functional Assessment of Chronic Illness Therapy; FACIT-Fatigue, The Functional Assessment of Chronic Illness Therapy – Fatigue Scale; Hb, haemoglobin; IVH, intravascular haemolysis; LDH, lactate dehydrogenase; LDL, low-density lipoprotein; LLN, lower limit of normal; MAVE, major adverse vascular event; PNH, paroxysmal nocturnal haemoglobinuria; RBC, red blood cell; SAE, serious adverse event; SD, standard deviation; SOC, system organ class; ULN, upper limit of normal; UTI, urinary tract infection; WBC, white blood cell.
References
FABHALTA® Summary of Product Characteristics.
ClinicalTrials.gov. Study of Efficacy and Safety of Twice Daily Oral LNP023 in Adult PNH Patients With Residual Anemia Despite Anti-C5 Antibody Treatment (APPLY-PNH). Available at: https://clinicaltrials.gov/study/NCT04558918. [Accessed February 2025]
Peffault de Latour R, et al. N Engl J Med 2024;390(11):994-1008.
Montan I, et al. Value Health 2018;21:1313–1321.
Novartis. Data on File. LNP023C1.
Novartis. Data on File. FA-11229368.
Kulasekararaj AG, et al. Blood Rev 2023;59:101041
ClinicalTrials.gov. Study of Efficacy and Safety of Twice Daily Oral Iptacopan (LNP023) in Adult PNH Patients Who Are Naïve to Complement Inhibitor Therapy (APPOINT-PNH). NCT04820530. Available at: https://clinicaltrials.gov/study/NCT04820530. [Accessed February 2025]
APPOINT-PNH Clinical Study Report. LNP023/iptacopan. CLNP023C12301.
Novartis. Data on File. FA-11229411.
UK | February 2025 | FA-11270168-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.

