Contact us
Need more information? Your local key account manager will be in contact shortly.
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
KESIMPTA is indicated for the treatment of adult patients with relapsing forms of multiple sclerosis with active disease defined by clinical or imaging features.1
For full safety information, please refer to KESIMPTA Summary of Product Characteristics (SmPC).1
KESIMPTA is an anti-CD20 mAb that acts on the immune system, specifically B-cells.1
MS is a complex, autoimmune condition defined by inflammation, demyelination and axonal damage in the central nervous system. This damage is caused by B-cells within the lymph nodes, which capture auto-antigens from neurons or their myelin sheaths and, upon presentation to T-cells, direct them to attack the body’s CNS tissue.2,3
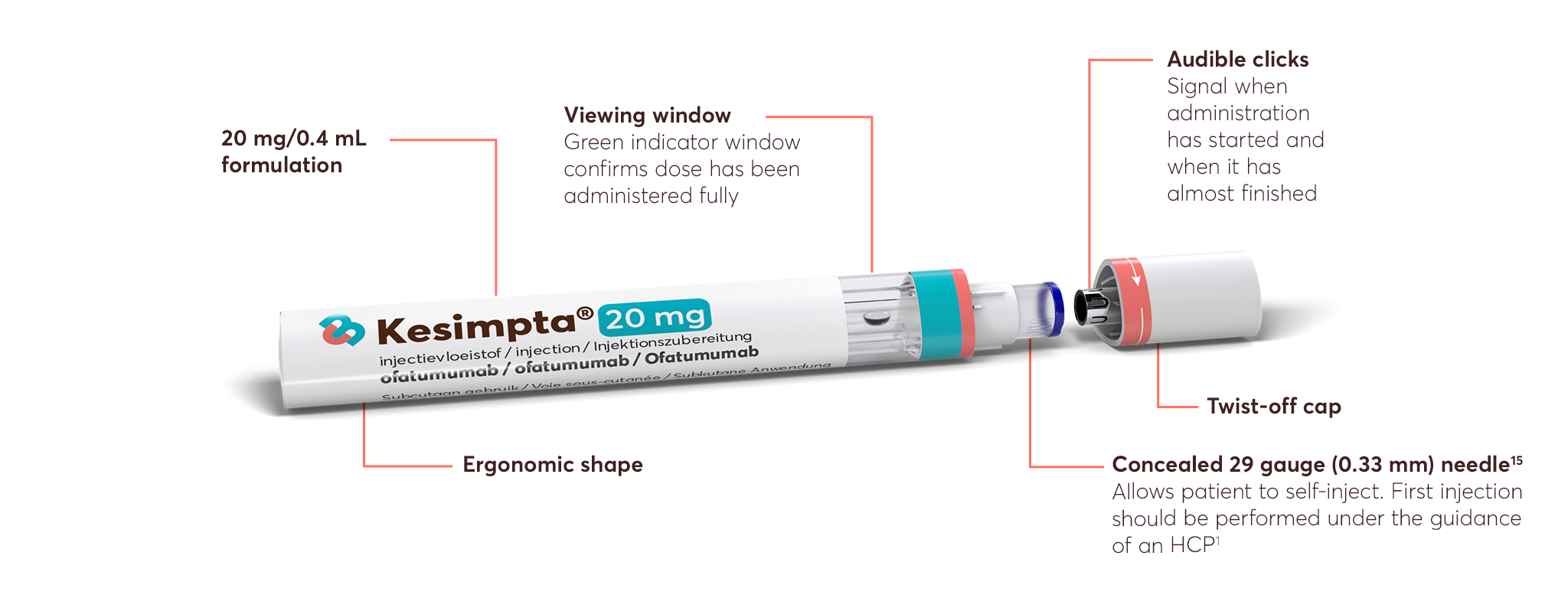
Following the initial dosing period, KESIMPTA offers a 1 minute-a-month self-administration (after preparation), at home or on the go, giving patients the independence to administer their treatment in an appropriate environment of their choice.*1,4
KESIMPTA is intended for patient self-administration, with initial guidance from an appropriately trained HCP.1 The flexibility to self-administer without the need for HCP involvement means dosing can occur at home or elsewhere appropriate outside the hospital setting. ‘1 minute a month’ refers to the time it takes for a patient to inject a full dose of KESIMPTA; based on stability data.4
In a US real-world survey:16
89.5% of patients found the KESIMPTA pen to be easy to use overall (n/N=94/105)‡
KESIMPTA is intended for patient self-administration subcutaneously, with initial guidance from an appropriately trained HCP.1
This flexibility to self-administer without the need for HCP involvement means dosing can occur at home or elsewhere appropriate outside the hospital setting.
Store in a refrigerator (2°C–8°C). Do not freeze. If necessary, KESIMPTA may be stored unrefrigerated for a single period of up to 7 days at room temperature (not above 30°C). If not used during this period, KESIMPTA can then be returned to the refrigerator for a maximum of 7 days.1
MS is a complex, autoimmune condition defined by inflammation, demyelination and axonal damage in the CNS. This damage is caused by B-cells within the lymph nodes, which capture auto-antigens from neurons or their myelin sheaths and, upon presentation to T-cells, direct them to attack the body’s CNS tissue.2,3
To learn more about the pathogenesis of MS and the role of B-cells in the disease, watch this short video.
KESIMPTA has been shown to act on B-cells in MS patients, targeting them and inducing cell lysis.1
KESIMPTA is an anti-CD20 monoclonal immunoglobulin G1 (IgG1) antibody that selectively targets and binds to the CD20 protein.1 This protein is expressed on the surface of the majority of B-cells, making it a valuable target for precise MS treatment.17 KESIMPTA is thought to work by selectively binding to sites on both the small and the large extracellular loops of CD20 protein on B-cells.17–19
The precise mechanism of action by which KESIMPTA exerts its therapeutic effects is unknown. To learn about how KESIMPTA targets B-cell subsets that facilitate inflammation, and how it causes CD20-inducing lysis of these B-cells, watch this short video.1
Induced lysis of CD20+ B-cells results in depletion through complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC). KESIMPTA has been shown to induce target cell death in both high and low CD20-expressing cells.1
The selective mechanism of action and precise delivery arising from the subcutaneous mechanism of action enables preferential depletion of B-cells in the lymph nodes.8,9
The preservation of B-cells in the spleen, as demonstrated by preclinical evidence, may contribute to immune function.10
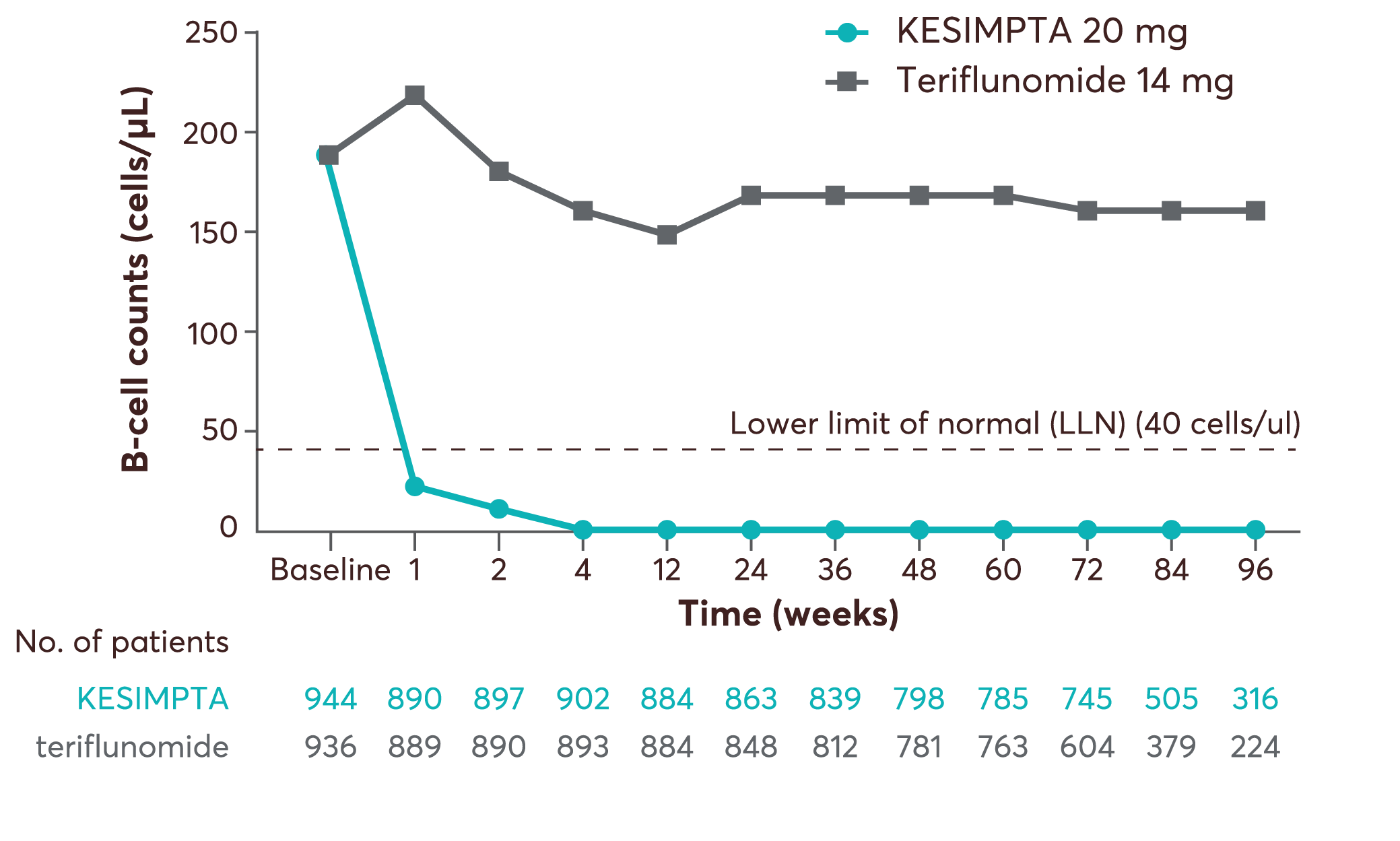
Dosing period from ASCLEPIOS I and II (pooled analysis)20
Adapted from Wiendl H, et al. 2020.20
In the RMS clinical studies using KESIMPTA 20 mg every 4 weeks, after an initial dose regimen of 20 mg on days 1, 7, and 14, administration resulted in a rapid and sustained reduction of B cells to below LLN (defined as 40 cells/µL) as early as two weeks after treatment initiation. Before initiation of the maintenance phase starting at week 4, total B-cell levels of <10 cells/µL were reached in 94% of patients, increasing to 98% of patients at week 12, and were sustained for as long as 120 weeks (i.e. while on study treatment).1
In the event of treatment discontinuation, B-cell repletion has also been studied in predictive models.
Simulated median and 90% prediction interval§21
Adapted from Yu H, et al. 2022.21
*Patient must take the pen out of the refrigerator about 15–30 minutes before self-administration to allow it to reach room temperature. Additional time is required to prepare the pen and cleanse. Store in a refrigerator (2°C–8°C). Do not freeze. If necessary, KESIMPTA may be stored unrefrigerated for a single period of up to 7 days at room temperature (not above 30°C). If not used during this period, KESIMPTA can then be returned to the refrigerator for a maximum of 7 days. Please refer to the SmPC for full administration details.1
†The recommended dose of KESIMPTA is 20 mg, which was chosen through dose modelling based on B-cell depletion results and Phase II data.1,12–14
‡Based on a cross-sectional survey of adult patients with RMS (N=105) in the US who self-administered KESIMPTA with the KESIMPTA pen within the previous 12 months. A total of seven attributes of the KESIMPTA pen were assessed, including ‘administration time’, ‘ease of monthly dosing schedule’ and ‘portability’.16
§Based on pharmacokinetic B-cell modelling (B-cell model: an indirect response model to describe the stimulation of B-cell lysis by free ofatumumab concentrations) of B-cell counts from 1486 patients with SC KESIMPTA administration and 25 patients with intravenous KESIMPTA administration across 5 pooled studies (MIRROR, OMS115102, ASCLEPIOS I & lI and APLIOS). Simulation is for subcutaneous route with pre-filled syringe and using Phase Ill dosage regimen.21 KESIMPTA is not licensed for intravenous administration; please see the SmPC for more details.
ADCC, antibody-dependent cellular cytotoxicity; CD, cluster of differentiation; CDC, complement-dependent cytotoxicity; CNS, central nervous system; HCP, healthcare professional; IgG, immunoglobulin G; IgM, immunoglobulin M; IV, intravenous; LLN, lower limit of normal; mAb, monoclonal antibody; MoA, mechanism of action; MS, multiple sclerosis; PK, pharmacokinetics; RMS, relapsing multiple sclerosis; RRMS, relapsing-remitting multiple sclerosis; SC, subcutaneous; SmPC, summary of product characteristics.
References
KESIMPTA (ofatumumab) Summary of Product Characteristics.
Pender MP, et al. Curr Allergy Asthma Rep 2007;(4):285–292.
Archelos JJ, et al. Ann Neurol 2000;47:694–706.
Novartis Data on file. Ofatumumab (OFA05). September 2022.
Hauser SL, et al. Neurol Ther 2023;12(5):1491–1515.
Wu F, et al. Pharm Res 2012;29(7):1843–1853.
Richter WF, et al. AAPS J 2012;14(3):559–570.
Torres JB, et al. Front Immunol 2022;13:814064.
Huck C, et al. J Neuroimmune Pharmacol 2019;14(4):709–719.
Theil D, et al. Front lmmunol 2019;10:1340.
Wiendl H, et al. ePresentation P9.010. American Academy of Neurology (AAN). 13–18 April 2024, Denver, US.
Sorensen PS, et al. Neurology 2014;82:573–581.
Bar-Or A, et al. Neurology 2018;90:e1805–e1814.
Savelieva M, et al. Poster P5.348. American Academy of Neurology (AAN). 22–28 April 2017, Boston, MA.
Novartis Data on File. Ofatumumab (OFA22). June 2024.
Ross AP, et al. Poster LB09. Consortium of Multiple Sclerosis (CMSC) Annual Meeting; 31 May–3 June 2023, Aurora, US.
Zhang B. mAbs 2009;1(4):326–331.
Gupta IV & Jewell RC. Ann N Y Acad Sci 2012;1263:43–56.
Martin R, et al. Eur J Immunol 2016;46:2078–2090.
Wiendl H, et al. ePresentation EPR3101. European Association of Neurology (EAN), 23–26, 2020. Virtual.
Yu H, et al. CNS Drugs 2022;36(3):283–300.
UK | January 2025 | 443403
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.