KESIMPTA resources
Don’t miss out on these resources to support you and your patients.
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
KESIMPTA is indicated for the treatment of adult patients with relapsing forms of multiple sclerosis (RMS) with active disease defined by clinical or imaging features.1
For full dosing and administration information, please refer to the KESIMPTA Summary of Product Characteristics (SmPC).1
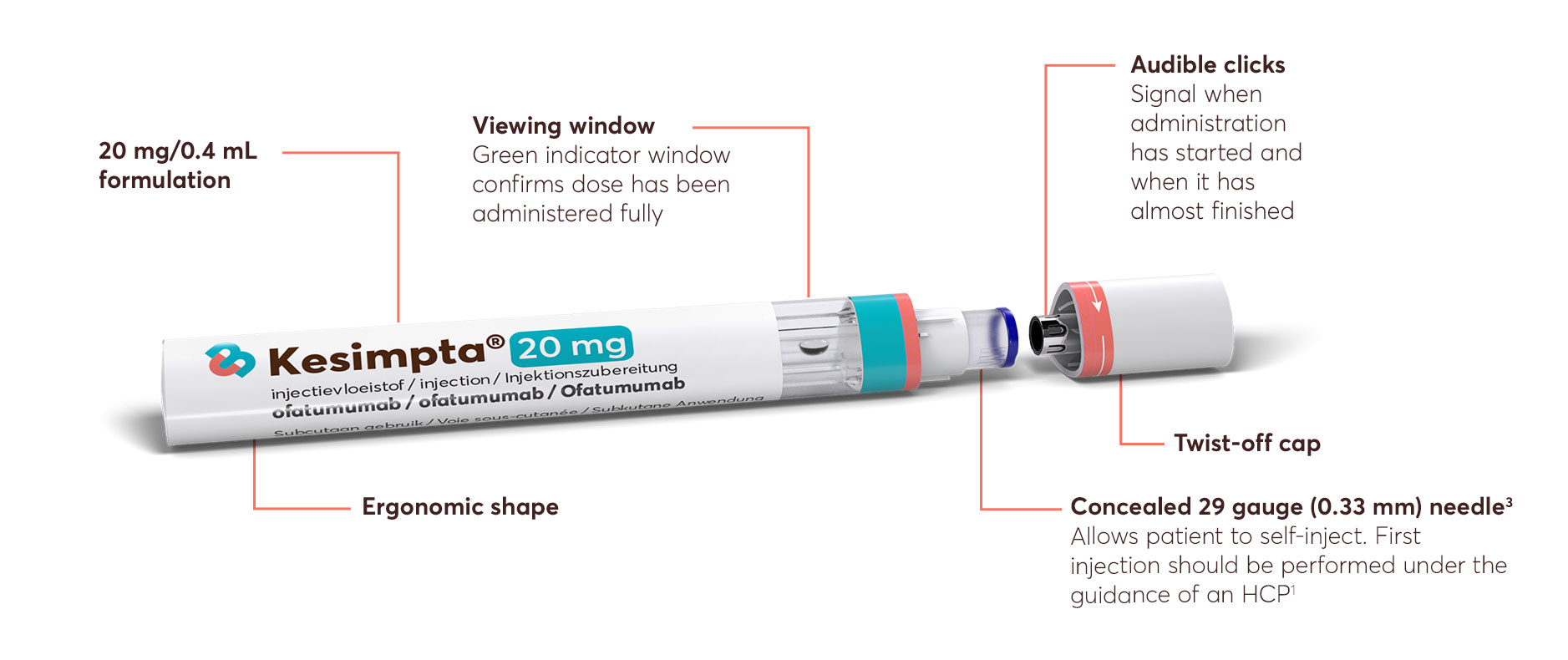
KESIMPTA is intended for patient self-administration, with initial guidance from an appropriately trained HCP.1 The flexibility to self-administer without the need for HCP involvement means dosing can occur at home or elsewhere appropriate outside the hospital setting. ‘1 minute a month’ refers to the time it takes for a patient to inject a full dose of KESIMPTA; based on stability data.2
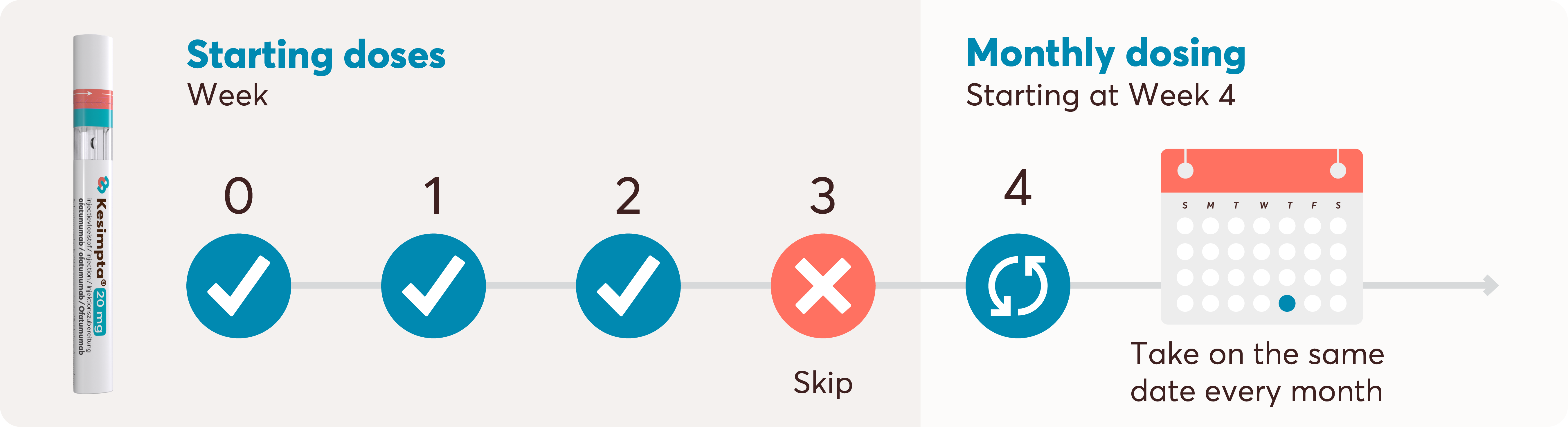
Initial doses of KESIMPTA are at Weeks 0, 1 and 2. The once-monthly dosing schedule begins at Week 4. All doses are 20 mg/0.4 mL formulation and are administered via subcutaneous injection.1
KESIMPTA is intended for patient self-administration with initial guidance of an appropriately trained HCP. After the initial dosing period, KESIMPTA 20 mg is self-administered once monthly at home or on the go.1 This flexibility to self-administer without the need for HCP involvement means dosing can occur at home or elsewhere appropriate outside the hospital setting.
Please see the SmPC for details on monitoring of patients with positive hepatitis B serology. Patients with active hepatitis B disease should not be treated with KESIMPTA.
To educate your patients about the KESIMPTA dosing schedule, you can direct them to a portal specifically designed for patients. Here, prescribed patients may access resources and support. The first injection of KESIMPTA should be performed under the guidance of a healthcare professional.
Before initiating treatment, KESIMPTA’s safety profile and KESIMPTA’s prescribing information should be reviewed.
KESIMPTA is the first and only self-administered once-monthly (20 mg), subcutaneous (SC) B-cell therapy for relapsing forms of multiple sclerosis (RMS) with active disease defined by clinical or imaging features.1
KESIMPTA is intended for patient self-administration with initial guidance from a healthcare professional (HCP). Please refer to the KESIMPTA Summary of Product Characteristics for further information.1
Watch this video to learn more about how to use KESIMPTA. This will help you teach your patients how to administer KESIMPTA with the Sensoready® pen after the initial dose.
Information for patients can be found on our patient resource page.
Please see the SmPC for details on monitoring of patients with positive hepatitis B serology. Patients with active hepatitis B disease should not be treated with KESIMPTA.
*Patient must take the pen out of the refrigerator about 15–30 minutes before self-administration to allow it to reach room temperature. Additional time is required to prepare the pen and cleanse. Store in a refrigerator (2°C–8°C). Do not freeze. If necessary, KESIMPTA may be stored unrefrigerated for a single period of up to 7 days at room temperature (not above 30°C). If not used during this period, KESIMPTA can then be returned to the refrigerator for a maximum of 7 days. Please refer to the SmPC for full administration details.1
†Based on a cross-sectional survey of adult patients with RMS (N=105) in the US who self-administered KESIMPTA with the KESIMPTA pen within the previous 12 months. A total of seven attributes of the KESIMPTA pen were assessed, including ‘administration time’, ‘ease of monthly dosing schedule’ and ‘portability’.4
‡Store in a refrigerator (2°C–8°C). Do not freeze. If necessary, KESIMPTA may be stored unrefrigerated for a single period of up to 7 days at room temperature (not above 30°C). If not used during this period, KESIMPTA can then be returned to the refrigerator for a maximum of 7 days. Please refer to the SmPC for full administration details.1
HCP, healthcare professional; MS, multiple sclerosis; RMS, relapsing forms of MS; SC, subcutaneous; SmPC, summary of product characteristics.
References
KESIMPTA (ofatumumab) Summary of Product Characteristics.
Novartis Data on File. Ofatumumab (OFA005). September 2022.
Novartis Data on File. Ofatumumab (OFA22). June 2024.
Ross AP, et al. Poster LB09. Consortium of Multiple Sclerosis Centers (CMSC) Annual Meeting; 31 May–3 June 2023, Aurora, US.
UK | January 2025 | 443398
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.