ASCLEPIOS clinical trials and efficacy
ASCLEPIOS clinical trials and efficacy
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
KESIMPTA®▼ (ofatumumab) is indicated for the treatment of adult patients with relapsing forms of multiple sclerosis with active disease defined by clinical or imaging features.1
For full safety information, please refer to the KESIMPTA Summary of Product Characteristics (SmPC).1
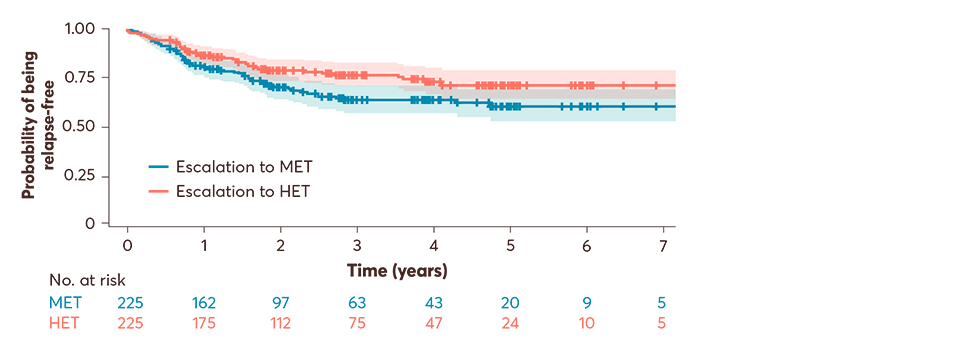
In a retrospective cohort study of patients with RRMS from the Swiss National Treatment Registry (2007–2023):2
450 patients with RRMS had been maintained on low-efficacy DMTs for ≥2 years and were subsequently escalated to either medium-efficacy DMTs (n=225) or high-efficacy DMTs (n=255; primary analysis)†‡
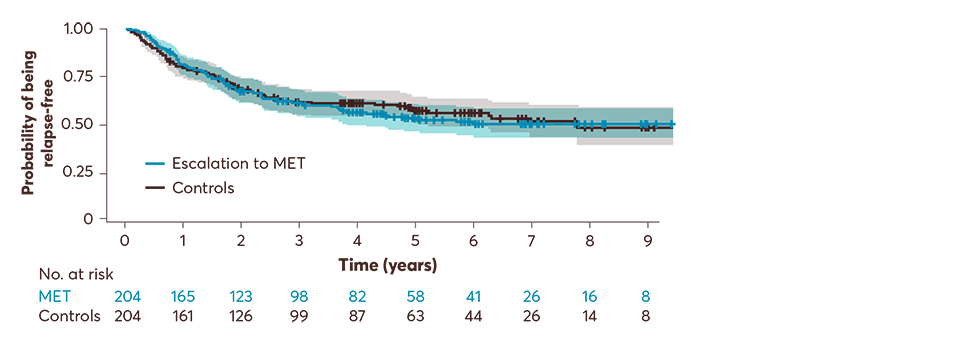
Each escalating group was then matched separately with control patients who remained on low-efficacy DMTs (secondary analysis)
KESIMPTA was not used in this study and therefore results must not be extrapolated.
Primary analysis: up to 7 years, escalation to high-efficacy DMTs was associated with an observed reduced risk of relapses vs medium-efficacy DMTs (HR: 0.67; 95% CI: 0.47–0.95; p=0.027). The median follow-up to pair-wise censoring was 1.9 years, during which 125 outcome events occurred; 73 (58%) in patients switching to MET and 52 (42%) in those switching to HET.2
Adapted from Müller J, et al. 2024.2
Secondary analysis: up to 9 years, escalation from low- to medium-efficacy DMTs did not change the risk of relapses when compared with control patients on low-efficacy DMTs who did not escalate treatment (HR: 1.19; 95% CI: 0.89–1.60, p=0.2). At a median follow-up of 2.9 years, relapses occurred in 87 (48.6%) of the patients escalating to MET and 92 (51.4%) patients in the control group.2
Adapted from Müller J, et al. 2024.2
*Better outcomes in terms of risk of relapse, ARR and EDSS.2–4
†To balance the groups for their baseline characteristics, the propensity of treatment escalation strategy was estimated using a multivariable logistic regression model with treatment allocation as outcome and age, sex, disease duration, EDSS, previous treatment, duration on the previous treatment, number of previous treatments, number of relapses in the prior 1 and 2 years, total number of prior relapses, and time since last relapse as independent variables. Nearest neighbour matching was used in a 1:1 ratio, with a calliper of 0.2 SD of the propensity score, without replacement. Patients who were escalated from low-efficacy to medium-efficacy treatment vs from low-efficacy treatment to high-efficacy treatment were matched first. Subsequently, each of these groups was matched separately with the controls.2
‡Low-efficacy DMTs included interferon beta-1a, peginterferon beta-1a, interferon beta-1b, glatiramer acetate, teriflunomide; medium-efficacy DMTs included fingolimod, dimethyl fumarate, ponesimod, ozanimod; and high-efficacy DMTs included ocrelizumab and natalizumab.2
ARR, annualised relapse rate; CI, confidence interval; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; HET, high-efficacy treatment; HR, hazard ratio; MET, medium-efficacy treatment; RR, rate ratio; RRMS, relapsing-remitting multiple sclerosis; SD, standard deviation; SmPC, summary of product characteristics.
References
KESIMPTA (ofatumumab) Summary of Product Characteristics.
Müller J, et al. Brain Behav 2024;14(5):e3498.
Harding K, et al. JAMA Neurol 2019;76(5):536–541.
He A, et al. Lancet Neurol 2020;19(4):307–316
UK | January 2025 | 443309
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.