KESIMPTA®▼ (ofatumumab) clinical studies
KESIMPTA is indicated for the treatment of adult patients with relapsing forms of multiple sclerosis with active disease defined by clinical or imaging features.1
ASCLEPIOS and ALITHIOS
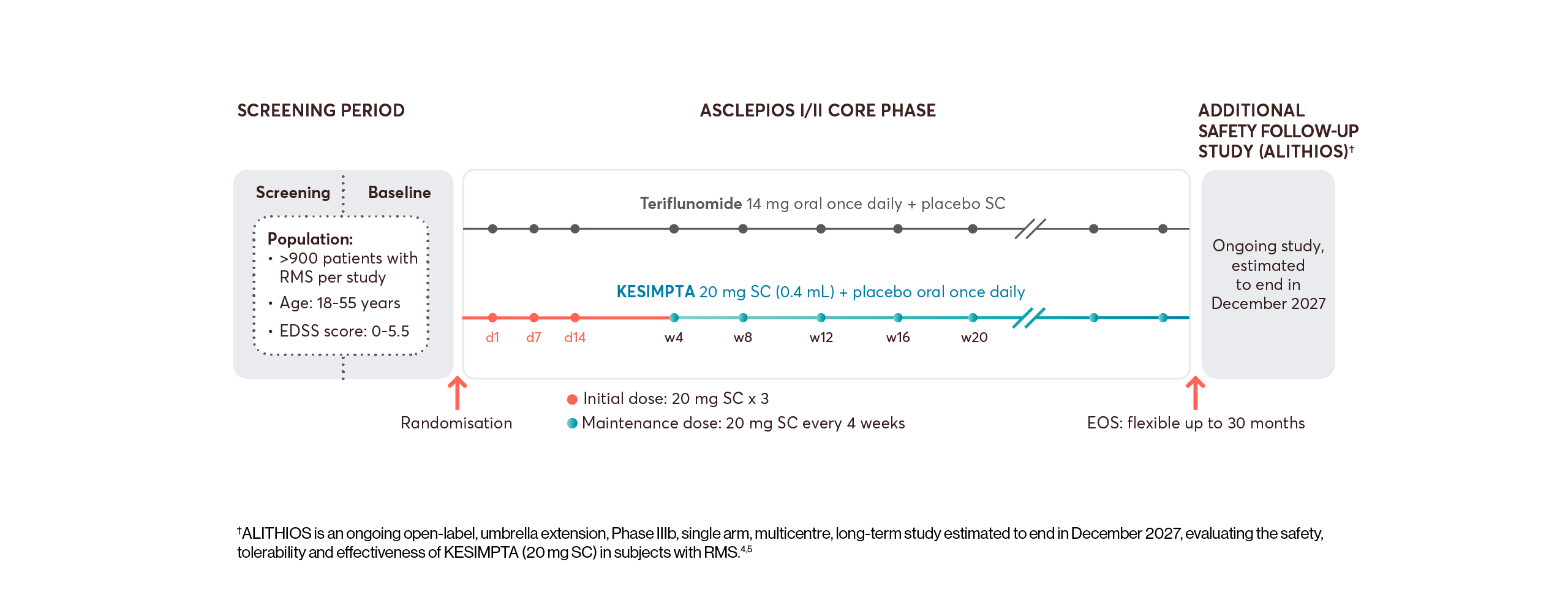
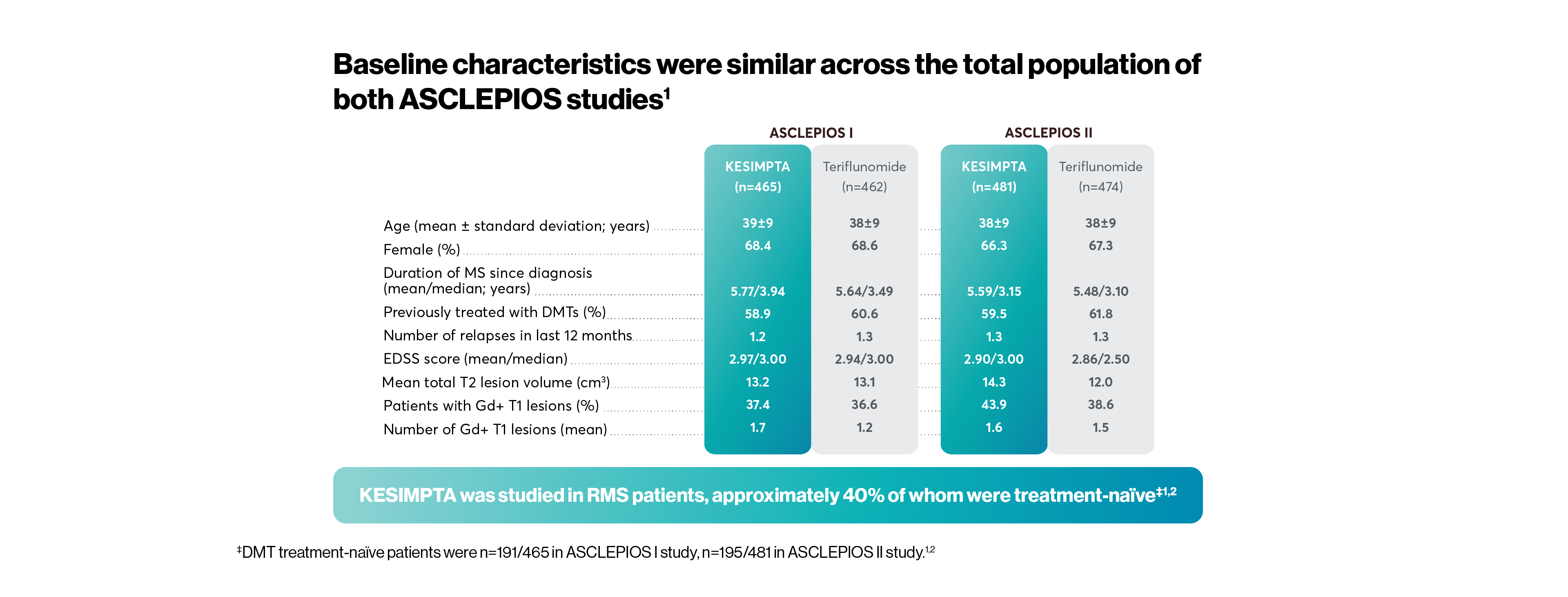
The efficacy and safety profile of KESIMPTA was initially studied vs an oral active comparator, teriflunomide, in two identically designed Phase III studies: ASCLEPIOS I and II (N=1882).*1,2
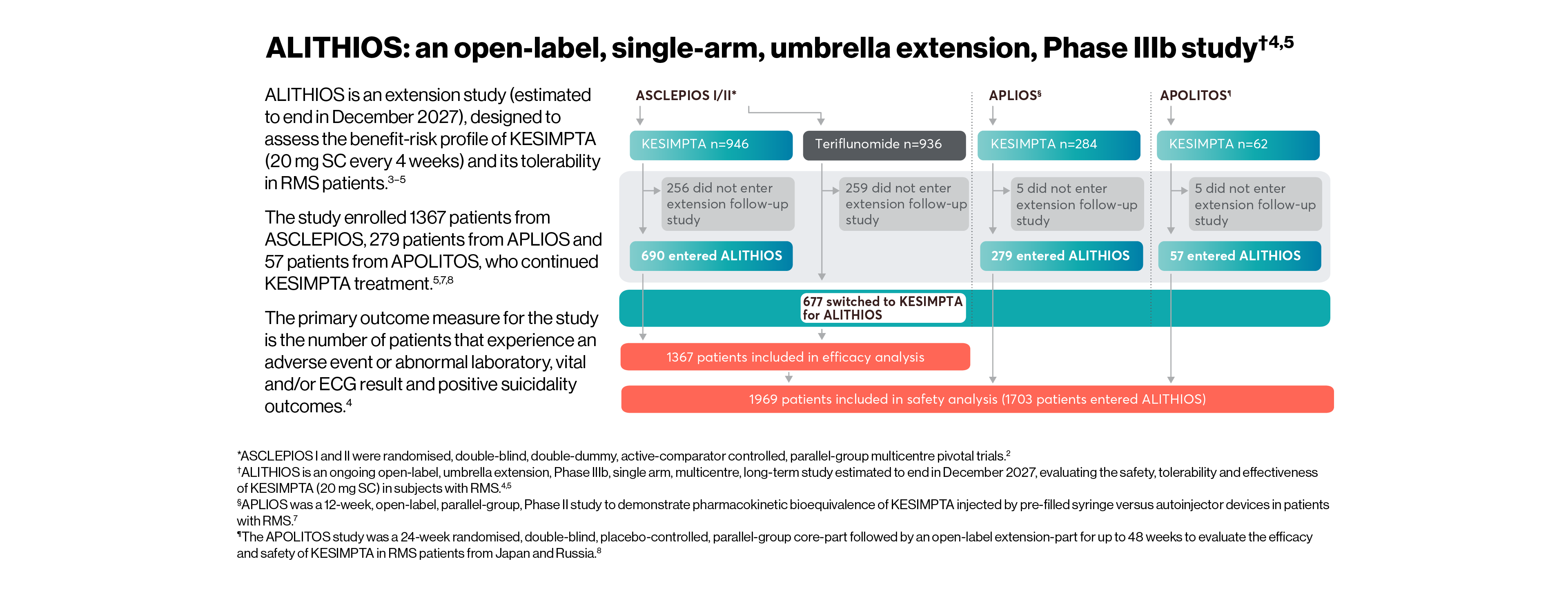
All patients who completed ASCLEPIOS I/II in the core period were eligible to enter ALITHIOS but could withdraw prior to treatment. The efficacy analysis set of ALITHIOS comprised all patients randomised to KESIMPTA or teriflunomide in ASCLEPIOS I/II, regardless of whether they completed/discontinued study treatment. In ALITHIOS, the safety analysis set comprised patients who received ≥1 dose of KESIMPTA in ASCLEPIOS I/II, APLIOS, APOLITOS, or ALITHIOS. ALITHIOS is an ongoing, open-label, extension study and was designed to assess the benefit-risk profile of KESIMPTA and its tolerability in RMS patients (N=1703); this is an ongoing study estimated to end in December 2027.†3–5
View the interactive study design for ASCLEPIOS and ALITHIOS below. To see more information, click on the arrows or scroll down, as appropriate.
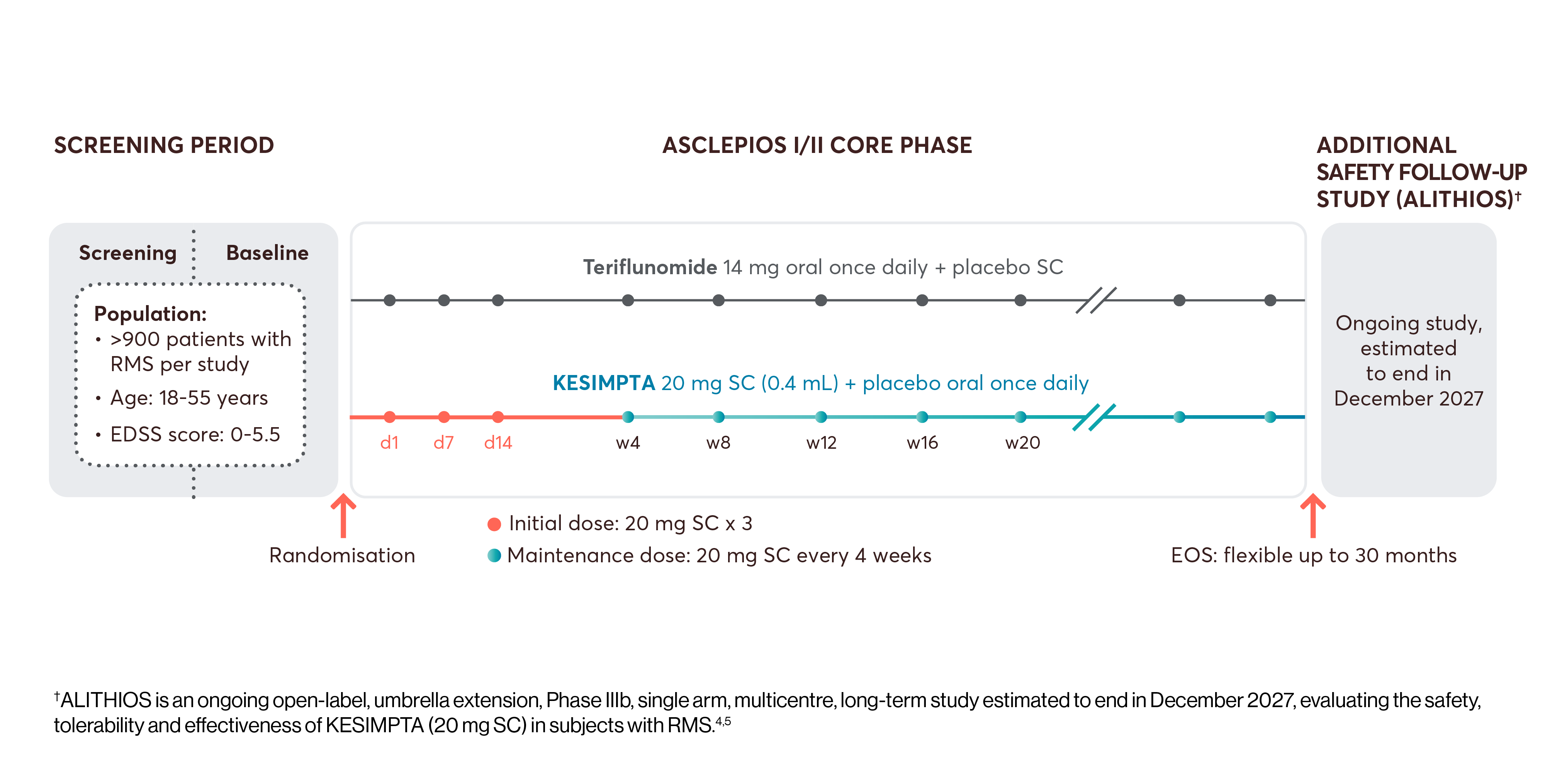

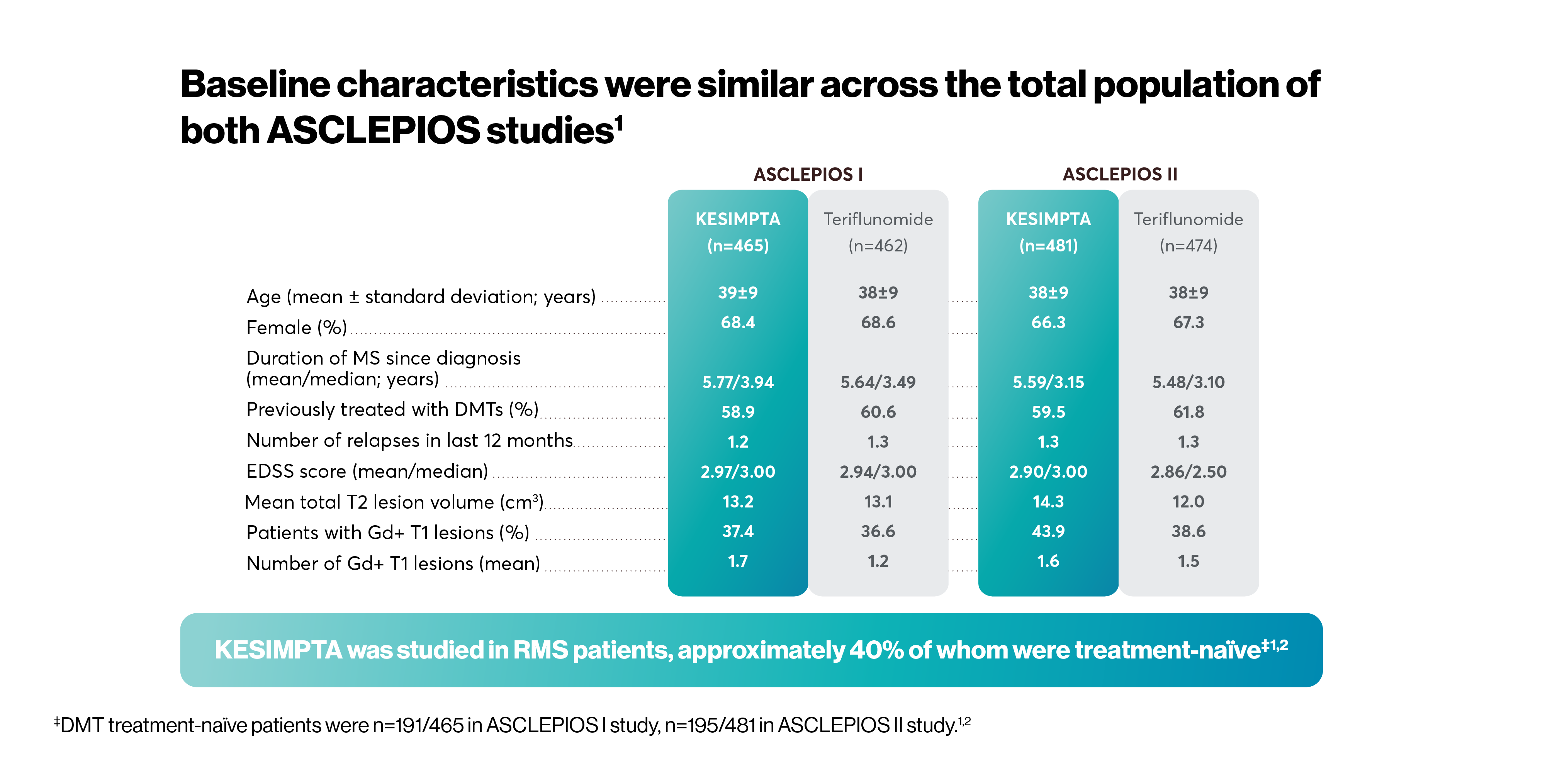

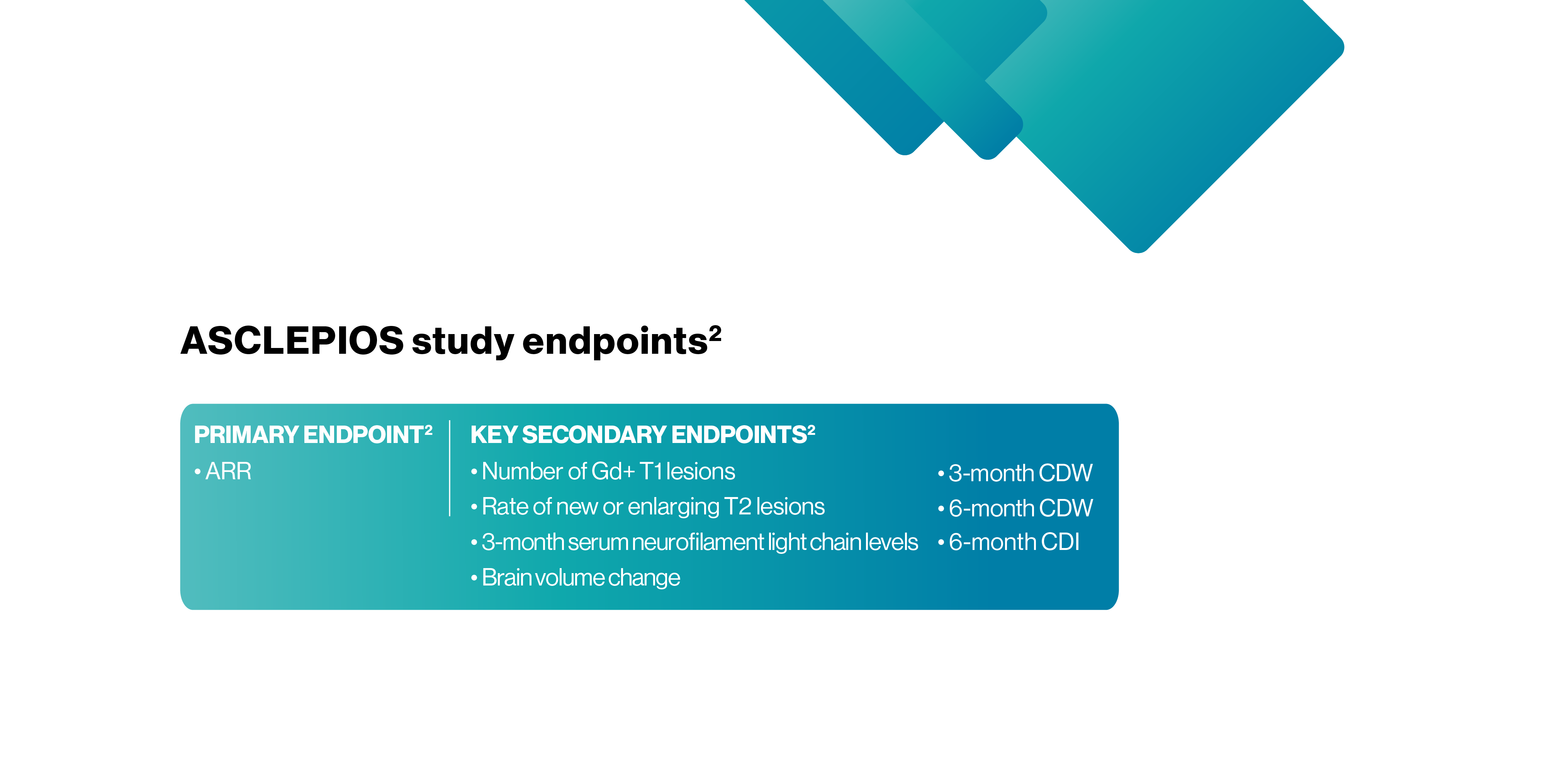

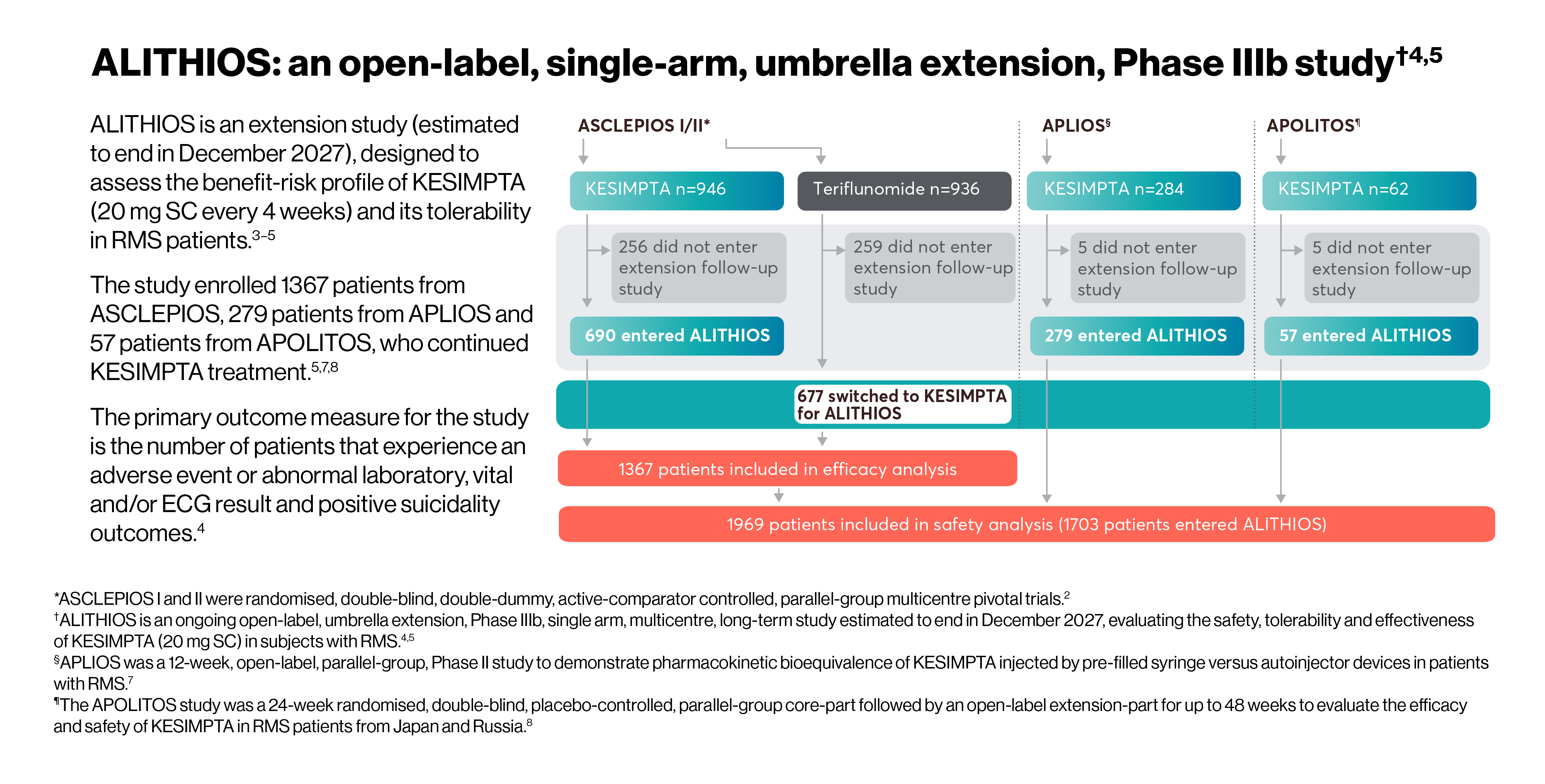
*ASCLEPIOS I and II were randomised, double-blind, double-dummy, active-comparator controlled, parallel-group multicentre pivotal trials.2
†ALITHIOS is an ongoing open-label, umbrella extension, Phase IIIb, single arm, multicentre, long-term study estimated to end in December 2027, evaluating the safety, tolerability and effectiveness of KESIMPTA (20 mg SC) in subjects with RMS.4,5
ARR, annualised relapse rate; CDI, confirmed disability improvement; CDW, confirmed disability worsening; DMT, disease-modifying therapy; ECG, electrocardiogram; EDSS, expanded disability status scale; EOS, end of study; Gd, gadolinium; Gd+, gadolinium enhancing; MRI, magnetic resonance imaging; MS, multiple sclerosis; RMS, relapsing forms of multiple sclerosis; RRMS, relapsing-remitting multiple sclerosis; SC, subcutaneous; SPMS, secondary progressive multiple sclerosis; W, week.
References
KESIMPTA (ofatumumab) Summary of Product Characteristics.
Hauser SL, et al. New Engl J Med 2020;383(6):546–557, and supplementary material.
Wiendl H, et al. American Academy of Neurology Annual Meeting, Denver, CO, USA; April 13–18, 2024, P9.010.
ClinicalTrials.gov. NCT03650114. Available at: https://clinicaltrials.gov/ct2/show/NCT03650114 [Accessed January 2025].
Hauser SL, et al. Mult Scler 2023;29(11–12):1452–1464.
O’Connor P, et al. Neurology 2016;86(10):920–930.
Bar-Or A, et al. Mult Scler 2022;28(6):910–924.
Kira JI, et al. Mult Scler 2022;28(8):1229–1238.
UK | January 2025 | 443372
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.