

Are people diagnosed with PV at risk of CV events?
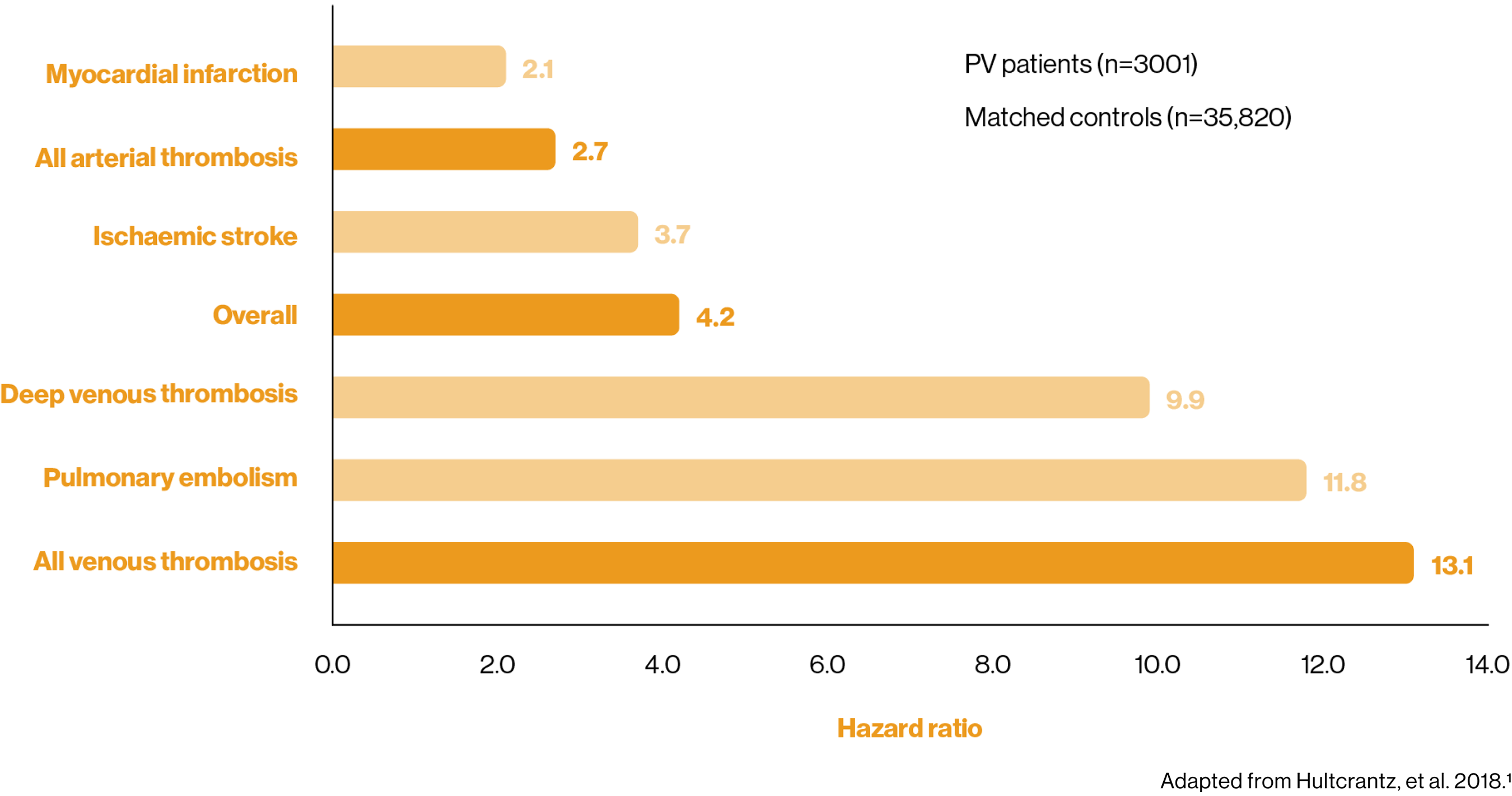
Patients with PV have a greater risk of thrombosis than the general population1
Arterial and venous thrombosis often trigger the PV diagnosis, but the elevated risk of a thromboembolic event remains at 3 months post-diagnosis.1,2
4-fold higher risk of overall thrombosis 3 months post-diagnosis vs matched control participants1
Population-based cohort study of patients with MPNs (n=9429), including patients with PV (n=3001).
Primary outcomes were rates of arterial and venous thrombosis.1
What is my patient’s risk category?
Age and a history of thrombosis are predictors of vascular complications in PV3
The BSH guidelines stratify patients according to their CV risk and recommend different treatment options for each set of patients:3
Category | Characteristics |
Low risk3 | Aged <65 years and no PV-associated thrombotic history |
High risk3 | Aged ≥65 years and/or prior PV-associated arterial or venous thrombosis |
The risks with high haematocrit
Uncontrolled HCT is associated with an increased risk of CV events and death4
In the large-scale multi-centre, prospective randomised CYTO-PV clinical trial, there was:4
The impact of blood counts
Elevated blood counts are associated with an increased risk of thrombosis in patients with PV5
Explore the increased risk of thrombosis when each component of blood is elevated:5
Risk of thrombosis remains in patients treated with HU6,7
Many patients can become resistant or intolerant to HU treatment.7 In order to maintain HCT control below 45%, patients treated with HU often require additional phlebotomies.6
Patients requiring three or more phlebotomies on top of HU treatment have an increased risk of thrombosis than those requiring two or less phlebotomies per year6
Recognise the signs of HU intolerance and resistance
Hydroxyurea is synonymous with/refers to hydroxycarbamide throughout.
*Data taken from an Italian multicentre study with 365 patients with PV. Patients were randomised 1:1 to receive aggressive therapy for an HCT target of <45% (low-HCT group) or less aggressive therapy for an HCT target of 45–50% (high-HCT group). Patients received phlebotomy, HU or both.4
†Event is defined as death from cardiovascular causes or thrombotic events (stroke, acute coronary syndrome, transient ischaemic attack, pulmonary embolism, abdominal thrombosis, deep-vein thrombosis, or peripheral arterial thrombosis).4
‡Definition of total CV events: death from cardiovascular causes or thrombotic events (stroke, acute coronary syndrome, transient ischaemic attack, pulmonary embolism, abdominal thrombosis, deep-vein thrombosis, or peripheral arterial thrombosis) and/or superficial-vein thrombosis.4
BSH, British Society for Haematology; CI, confidence interval; CV, cardiovascular; HCT, haematocrit; HR, hazard ratio; HU, hydroxyurea; MPN, myeloproliferative neoplasms; PV, polycythaemia vera.
References
Hultcrantz M, et al. Ann Intern Med 2018;168:317–325.
Griesshammer M, et al. Ann Hematol 2019;98:1071–1082.
McMullin F, et al. Br J Haematol 2019;184:176–191.
Marchioli R, et al. N Engl J Med 2013;368:22–33.
Gerds AT, et al. Oral presentation at the American Society of Hematology (ASH) annual meeting 2021; abstract 239.
Alvarez-Larrán A, et al. Haematologica 2017;102:103–109.
Alvarez-Larrán A, et al. Blood 2012;119:1363–1369.
UK | December 2024 | FA-11213864
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.







