

Treating PV appropriately in the long term
The British Society for Haematology recommends a variety of first-, second- and third-line treatments for patients with PV.1 Click here to see the BSH guideline recommendations.
Patients can become intolerant or resistant to conventional therapies2
HU is often used as a first-line cytoreductive treatment in high-risk patients.2 For patients under the age of 60, interferon* can be recommended instead.1,3,4
Switching to a second-line treatment may need to be considered, depending on individual patient factors.4 Up to 20.7% of patients with PV may become intolerant or resistant to HU treatment, which may lead to increased mortality.†5–7
Use the modified ELN recommendations to identify intolerance and resistance.4
*Not all forms of interferon therapies are licensed for the treatment of PV. Please refer to individual summary of product characteristics prior to prescribing.
Modified ELN criteria to help identify HU resistance and intolerance:4
Intolerance
Haematologic toxicities
Absolute neutrophil count <1.0 × 109/L
Platelet count <100 × 109/L
Haemoglobin <10 g/dL
Non-haematological toxicities
Fever
Manifestations of the mucous membrane
Gastrointestinal symptoms
Pneumonitis
Leg ulcers

Resistance
Thrombosis or bleeding
Unacceptable number of phlebotomies to keep HCT <45%‡
Persisting disease-related symptoms
Platelet count >400 × 109/L and/or white blood cell count >10 × 109/L‡
No reduction of splenomegaly or a reduction <50%‡
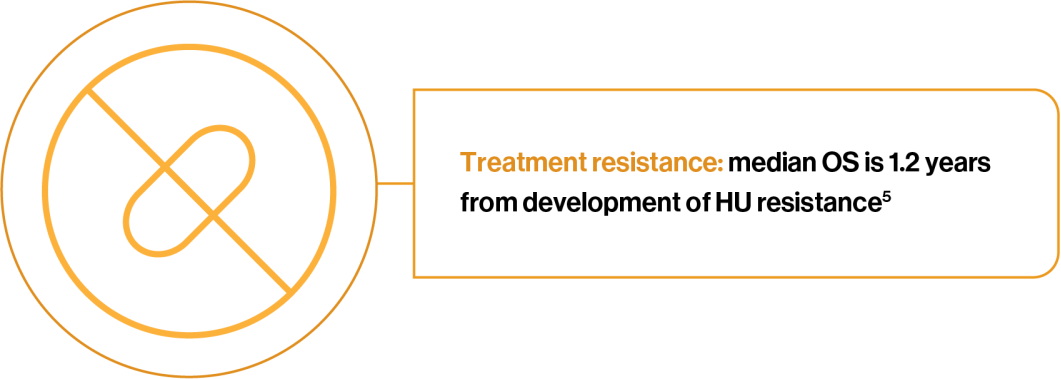
HU intolerance or resistance is associated with reduced survival, increased risk of thrombosis and reduced quality of life5,6,8–10
HU resistance decreases median OS5
The development of HU resistance, as defined by the ELN criteria, was assessed in patients with PV that were treated with HU for a median time of 4.4 years (n=261). Patients that developed HU resistance, compared to those that did not, experienced a:5
A failure to keep HCT levels below 45% is associated with an increase in CV-related mortality or thrombotic events8
The CYTO-PV study was a large-scale, multicentre, prospective, randomised clinical trial comparing the efficacy of phlebotomy, HU or both for maintaining a HCT target of less than 45%, as compared with maintaining a target of 45–50%, for the prevention of thrombotic events in patients with polycythaemia vera (n=365). The protocol dictated that the HCT target to which patients were randomly assigned in a 1:1 ratio had to be maintained during the course of the study. The primary composite endpoint was the time until death from cardiovascular causes or major thrombotic events.§8
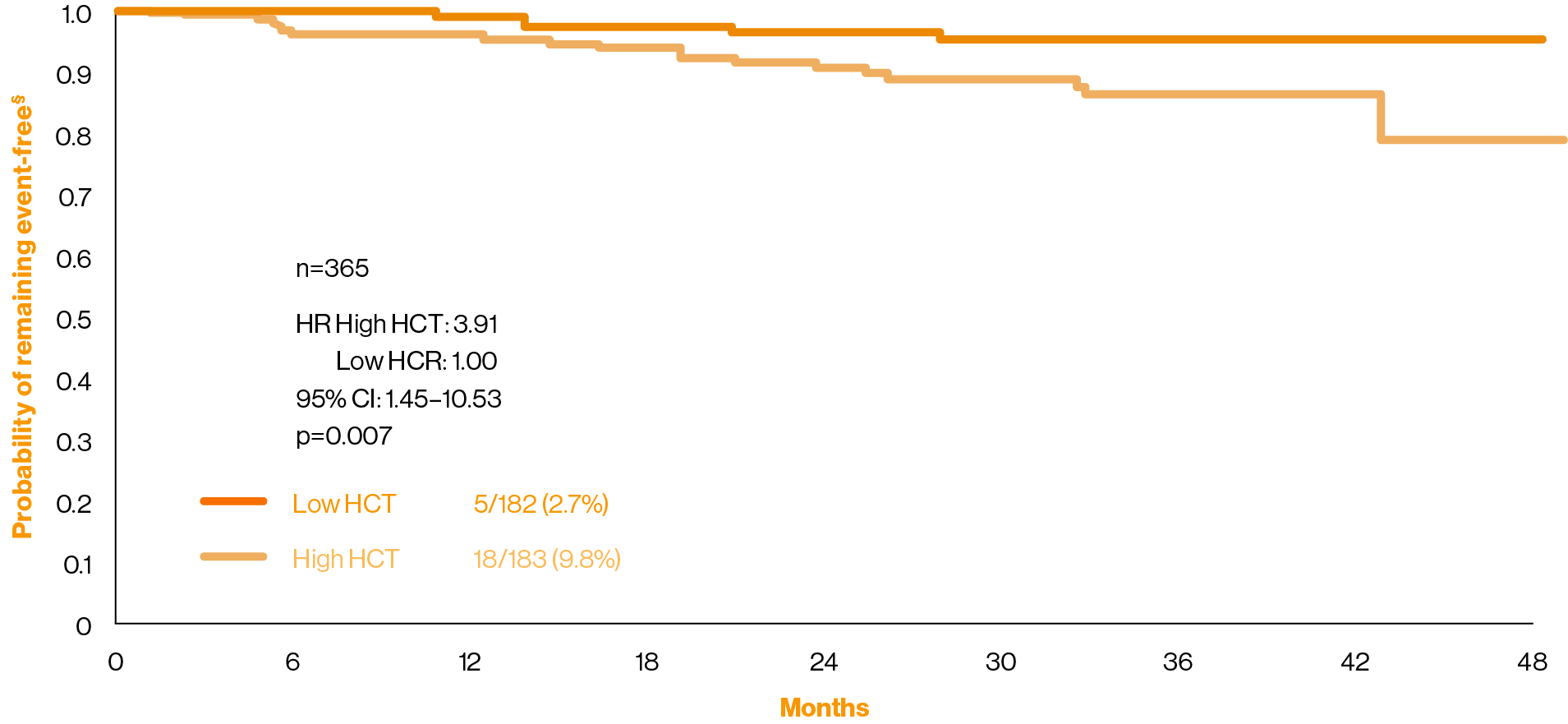
Adapted from Marchioli, et al. 2013.8

Skin toxicities linked with HU intolerance can lead to treatment discontinuation9
In a prospective, non-interventional study of skin alterations in 151 patients with MPNs (n=55 with PV) who gave informed consent:9
What treatment is recommended if patients become intolerant or resistant?
Patients with PV are recommended to switch to a second-line cytoreductive therapy if they meet the criteria for intolerance or resistance.3 The 2021 ELN recommendations for treatment suggest that treatment choice should be based on individual clinical features:3
Age
Spleen size
Symptoms
History of skin cancers
Patient preference
Download the Hydroxyurea Intolerance and Resistance Guide for further information on signs and treatment
How does living with PV affect patients’ daily lives?
Hydroxyurea is synonymous with/refers to hydroxycarbamide throughout.
†HU resistance or intolerance was defined using the 2010 ELN criteria.7,11 In a retrospective analysis of 106 patients with PV in Belgium, when using the original and modified ELN criteria, 20.7% and 39.6% of patients were resistant or intolerant to HU, respectively.4,7,11
‡After 3 months of at least 2 g per day of HU or the maximum tolerated dose.5
§Event is defined as death from cardiovascular causes or thrombotic events (stroke, acute coronary syndrome, transient ischaemic attack, pulmonary embolism, abdominal thrombosis, deep-vein thrombosis, or peripheral arterial thrombosis).8
BSH, British Society for Haematology; CI, confidence interval; CV, cardiovascular; ELN, European LeukemiaNet; HCT, haematocrit; HR, hazard ratio; HU, hydroxyurea; IFN-alpha, interferon-alpha; MPN, myeloproliferative neoplasms; OS, overall survival; PV, polycythaemia vera; QoL, quality of life.
References
McMullin F, et al. Br J Haematol 2019;184:176–191.
Raman I, et al. Leuk Lymphoma 2021;62:2310–2319.
Marchetti M, et al. Lancet Haematol 2022;9:e301–e311.
McMullin F, et al. Br J Haematol 2016;172:337–349.
Alvarez-Larrán A, et al. Blood 2012;119:1363–1369.
Alvarez-Larrán A, et al. Br J Haematol 2016;172:786–793.
Demuynck T, et al. Ann Hematol 2019;98:1421–1426.
Marchioli R, et al. N Engl J Med 2013;368:22–33.
Stegelmann FA, et al. EHA Congress 2017, 22–25 June; Madrid, Spain. Abstract E1335.
Harrison CN, et al. Ann Hematol 2017;96:1653–1655.
Barosi G, et al. Br J Haematol 2010;148:961–963.
UK | February 2025 | FA-11213873
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.




