

SCEMBLIX®▼ (asciminib) is indicated for the treatment of adult patients with Philadelphia chromosome-positive chronic myeloid leukaemia (Ph + CML) in chronic phase (CP), previously treated with two or more tyrosine kinase inhibitors, and without a known T315I mutation.1
Patient monitoring and management
Regular monitoring is important to assess treatment benefits and inform the decision to switch2,3
Monitoring milestones of BCR-ABL1 transcript levels by the IS (international scale) at 3, 6 and 12 months is essential to determine treatment interventions.2,3
In later lines of treatment, acceptable response cannot be formally defined, but a BCR-ABL1IS >1% is insufficient for optimal survival.2,3
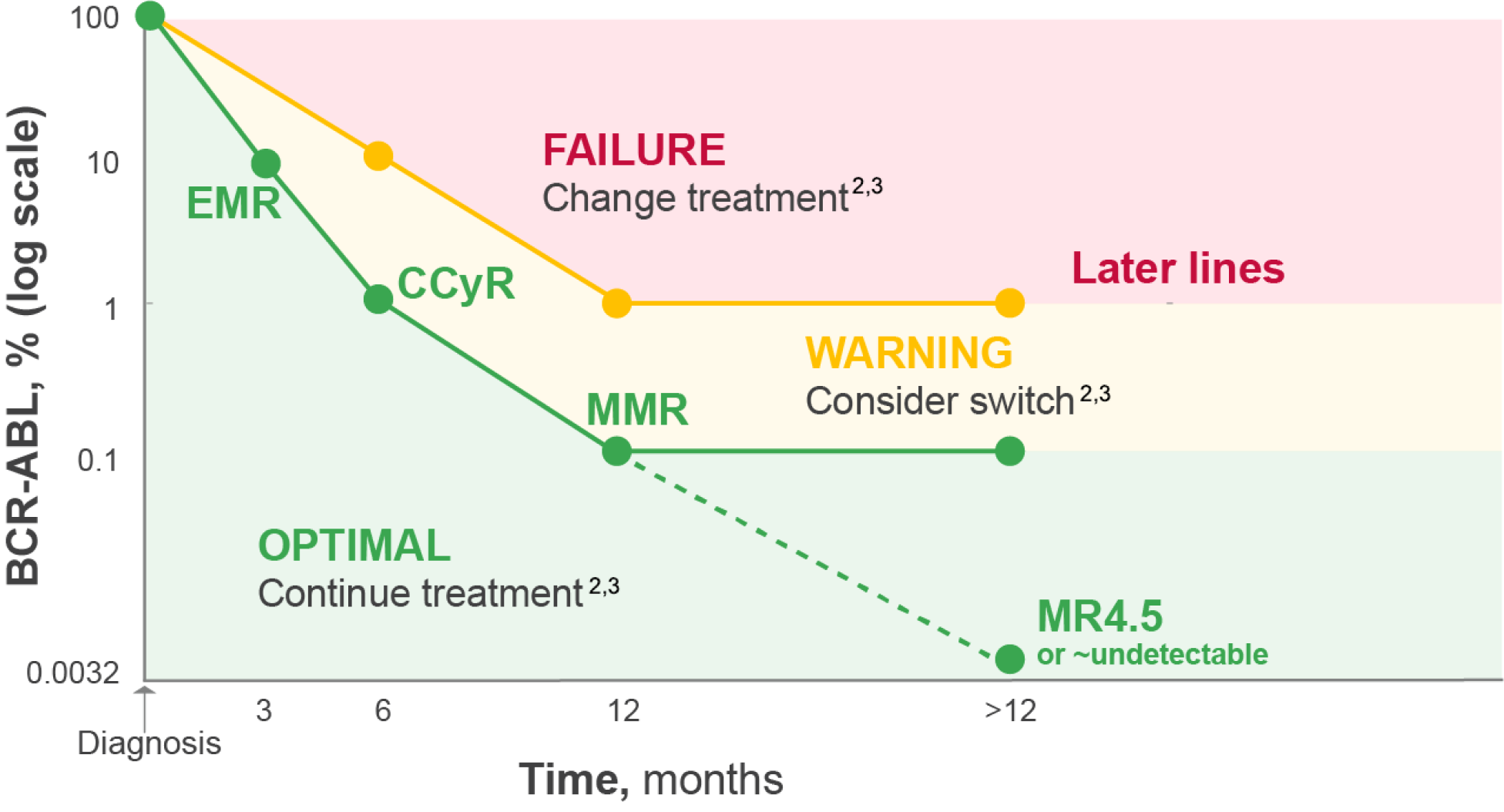
Adapted from Hocchaus A, et al. 2020 and Smith G, et al. 2020.2,3
For full details on monitoring, please see the ELN 2020 Guidelines and the SCEMBLIX SmPC.
Even after MMR is achieved, monitoring of MMR should continue every 3–6 months2,3
Make monitoring and management effective
BCR-ABL, breakpoint cluster region and Abelson murine leukaemia viral oncogene homologue; CCyR, complete cytogenetic remission; EMR, early molecular response; IS, international scale; MMR, major molecular response; MR, molecular response.
For further information, please refer to the Summary of Product Characteristics.
References:
SCEMBLIX (asciminib) Summary of Product Characteristics.
Hochhaus A, et al. Leukemia 2022;34:996-984.
Smith G, et al. Br J Haematol 2020;191(2):171–193.
UK | November 2024 | FA-11311678
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.

