

SCEMBLIX®▼ (asciminib) is indicated for the treatment of adult patients with Philadelphia chromosome-positive chronic myeloid leukaemia (Ph + CML) in chronic phase (CP), previously treated with two or more tyrosine kinase inhibitors, and without a known T315I mutation.1
Mechanism of action (MOA)
SCEMBLIX®▼ (asciminib) is the first and only STAMP inhibitor2–4
Learn how the unique MOA of SCEMBLIX enhances its specificity in treating CML.2
SCEMBLIX targets a different site on BCR-ABL1 – the myristoyl pocket2–4
In people who do not have CML, the myristoyl pocket is occupied by the N-terminal portion of ABL1, maintaining the protein in an inactive conformation.5,6
In BCR-ABL1, the myristoyl pocket is vacant, activating the kinase.5,6
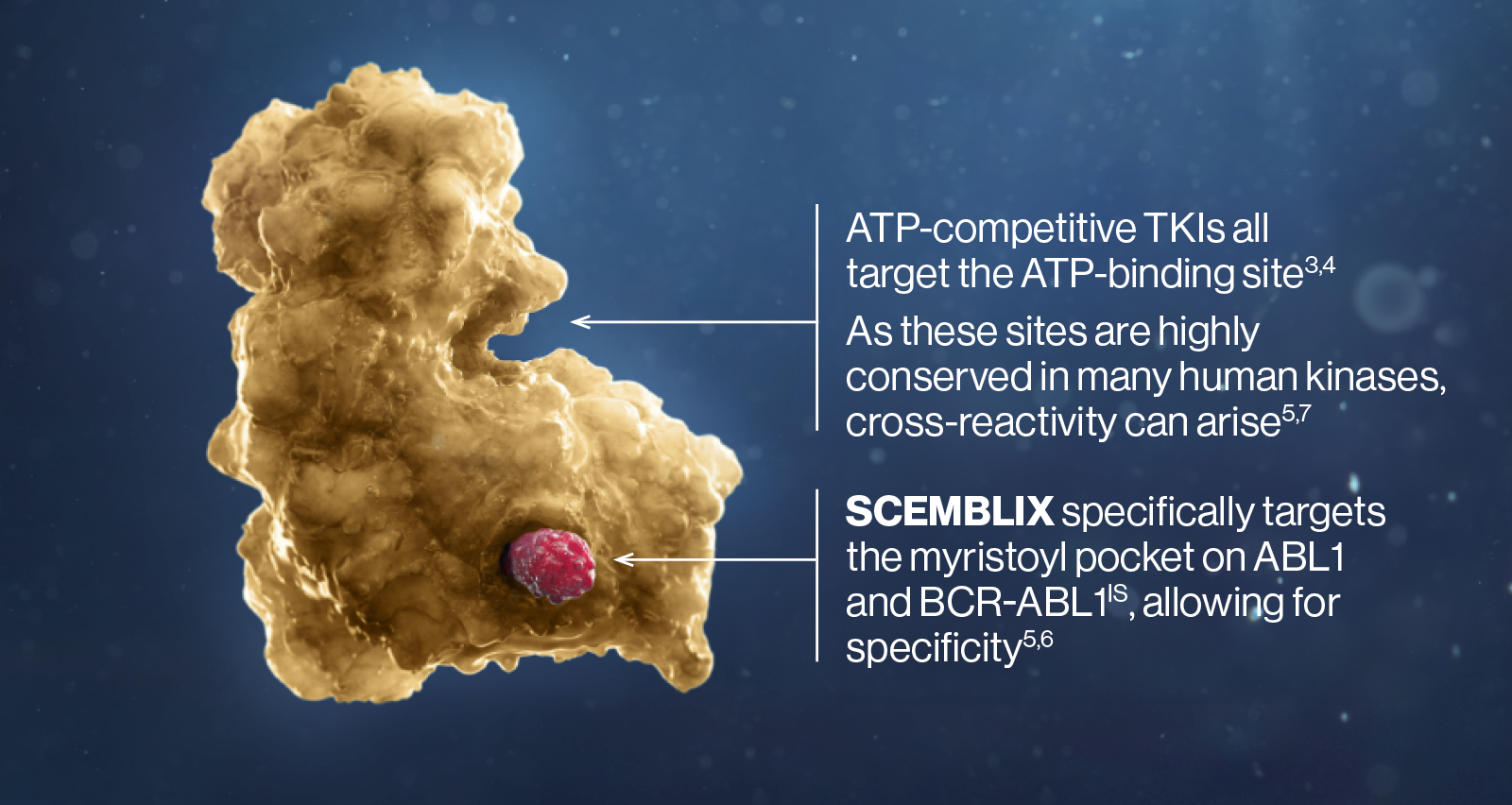
SCEMBLIX is a first-in-class STAMP inhibitor. Binding specifically to the myristoyl pocket, it potently inactivates BCR-ABL1 via allosteric inhibition.2
With its unique MOA, SCEMBLIX offers a different approach for treating CML5–7
SCEMBLIX demonstrated superior efficacy and a favourable safety profile vs bosutinib at Week 242
ATP, adenosine triphosphate; BCR-ABL, breakpoint cluster region and Abelson murine leukaemia viral oncogene homologue; CI, confidence interval; CML, chronic myeloid leukaemia; CP, chronic phase; MMR, major molecular response; MOA, mechanism of action; Ph + CML, Philadelphia chromosome-positive chronic myeloid leukaemia; STAMP, specifically targeting the ABL1 myristoyl pocket; TKI, tyrosine kinase inhibitor.
For further information, please refer to the Summary of Product Characteristics.
References:
SCEMBLIX (asciminib) Summary of Product Characteristics.
Réa D, et al. Blood 2021;138(21):2031–2041.
Redaelli S, et al. J Clin Oncol 2009;27(3):469–471.
Schoepfer J, et al. J Med Chem 2018;61(18):8120–8135.
Hughes TP, et al. N Engl J Med 2019;381(24):2315–2326.
Manley PW, et al. Leuk Res 2020;98:106458.
Iacob RE, et al. PLoS One 2011;6(1):e15929.
UK | November 2024 | FA-11311670
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.


