

SCEMBLIX®▼ (asciminib) is indicated for the treatment of adult patients with Philadelphia chromosome-positive chronic myeloid leukaemia (Ph + CML) in chronic phase (CP), previously treated with two or more tyrosine kinase inhibitors, and without a known T315I mutation.1
Efficacy
SCEMBLIX®▼ (asciminib) showed superior efficacy vs bosutinib as early as Week 241,2
ASCEMBL is the first head-to-head Phase III study comparing a STAMP inhibitor vs an ATP-competitive TKI.2,3
A multicentre, randomised, active-controlled and open-label Phase III study of SCEMBLIX vs bosutinib, assessing MMR at 24 and 96 weeks, and other endpoints including time to and duration of MMR, CCyR and safety profile.2
The ASCEMBL trial population was not restricted to Ph+ patients with CML-CP.2 SCEMBLIX is indicated in adults with Ph+ CML-CP previously treated with two or more TKIs and without a known T315I mutation.1
SCEMBLIX nearly doubled the MMR rate vs bosutinib at Week 24 (primary endpoint)2,4
MMR at Week 24
Primary endpoint2,4
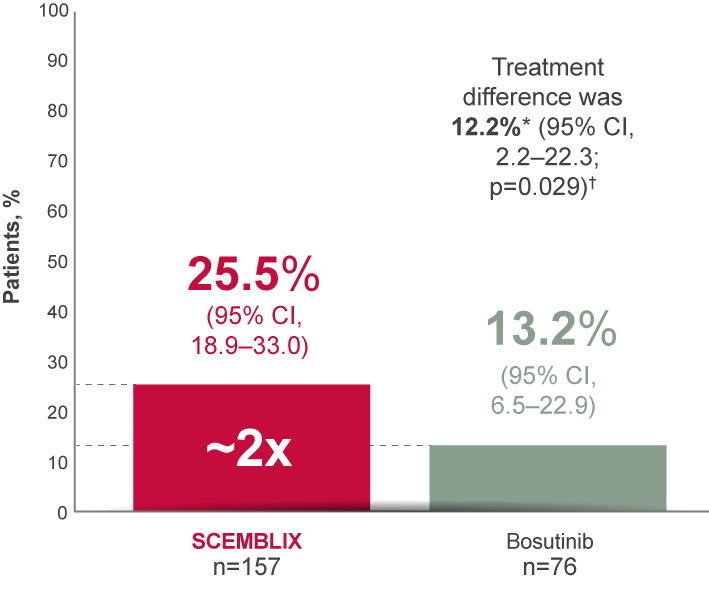
*On adjustment for the baseline major cytogenetic response status.
†Cochran–Mantel–Haenszel two-sided test stratified by baseline major cytogenetic response status.
Adapted from Réa D, et al. 20212 and Mauro M, et al. 2023.4
Achieving MMR has been associated with more favourable long-term outcomes, including survival and progression-free survival.2,5,6
Key secondary endpoint
Reported after a follow-up of 2.3 years2,4
MMR at Week 962,4
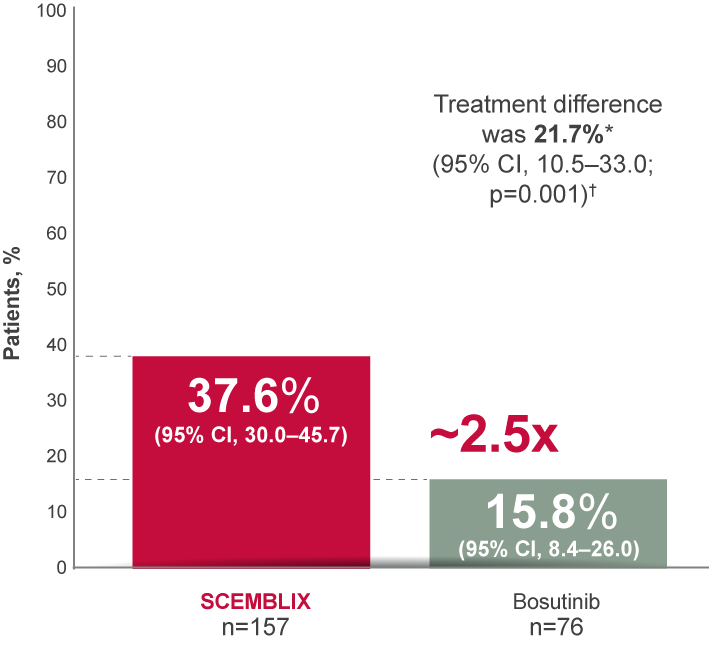
*On adjustment for the baseline major cytogenetic response status.
†Cochran–Mantel–Haenszel two-sided test stratified by baseline major cytogenetic response status.
Adapted from Réa D, et al. 20212 and Mauro M, et al. 2023.4
MMR continued to be higher with SCEMBLIX vs bosutinib at Week 96.2,4
End of study treatment
MMR at Week 1562,4
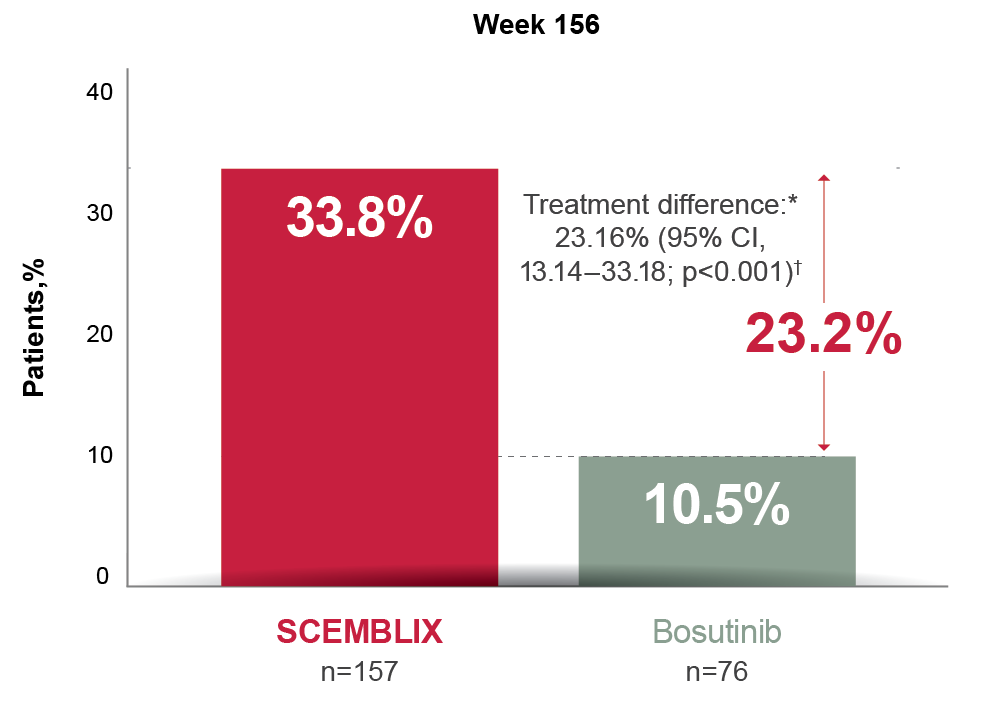
*On adjustment for the baseline major cytogenetic response status.
†Cochran–Mantel–Haenszel two-sided test stratified by baseline major cytogenetic response status.
Adapted from Réa D, et al. 2021,2 and Mauro M, et al. 2023.4
More patients receiving SCEMBLIX may yield CCyR status over time vs bosutinib1
Please note that p values were not formally tested for this analysis
CCyR at Week 24*1,2
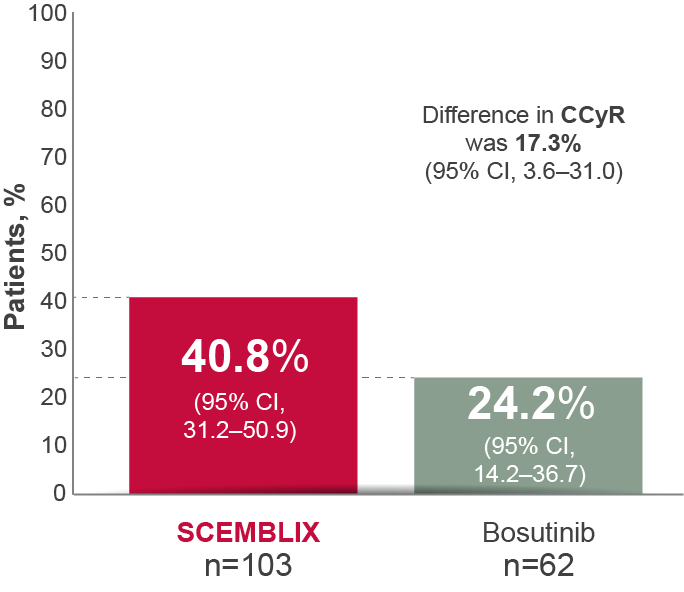
CCyR at Week 96*1–3
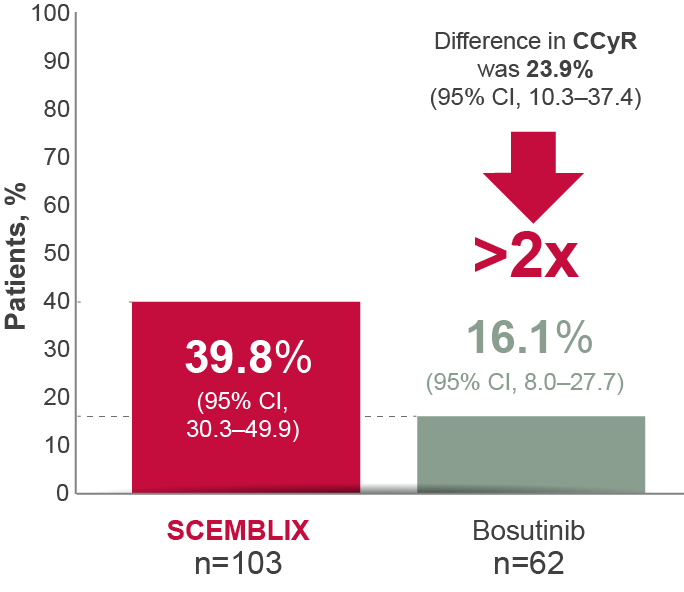
*CCyR analysis based on 103 of 157 patients (65.6%) receiving SCEMBLIX and 62 of 76 (81.6%) receiving bosutinib who did not have this level of response at baseline. Key secondary efficacy and safety results were reported after a median follow-up of 2.3 years (16.5 months of additional follow-up since primary analysis).2
Adapted from Réa D et al. 2021,2 Hochhaus A et al. 20233 and the SCEMBLIX SmPC.1
CCyR is a predictor of better long-term outcomes.7
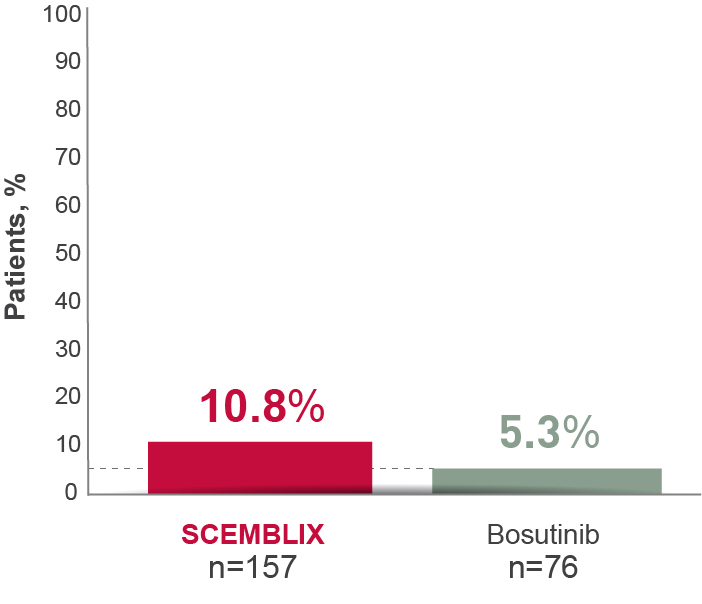
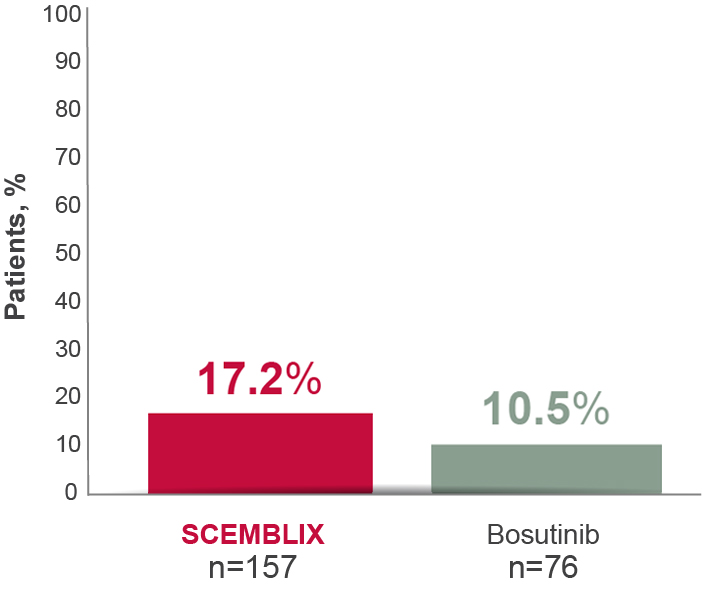
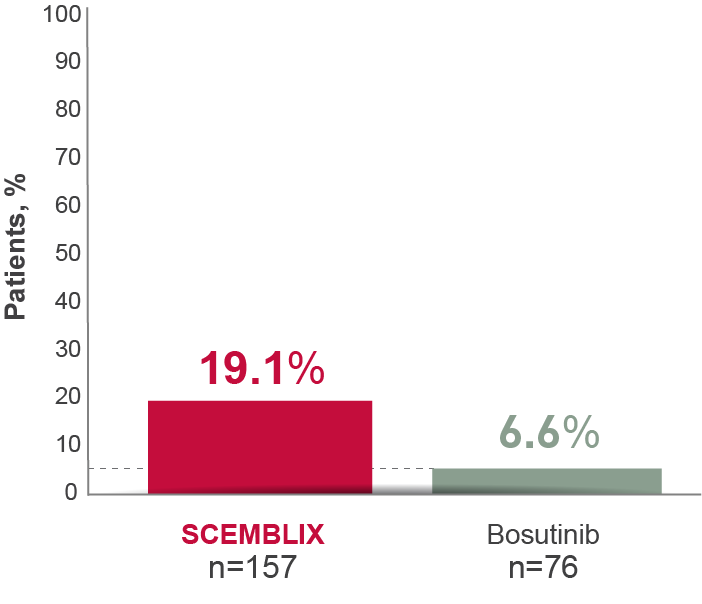
With SCEMBLIX, more patients achieved deep MR vs bosutinib at Week 24 through to Week 1562,8
No statistical analysis was available at the time of publication
MR4 at week 242,8
MR4 at week 968
MR4 at Week 1568
Adapted from Réa D, et al. 20212 and Mauro M, et al. 2023.8
Deep molecular response is defined as MR4 (BCR-ABL1IS ≤0.01%) or better (with MR4.5 BCR-ABL1IS ≤0.0032%).5
SCEMBLIX consistently increased cumulative MMR vs bosutinib over time2,4,8
Cumulative incidence of MMR
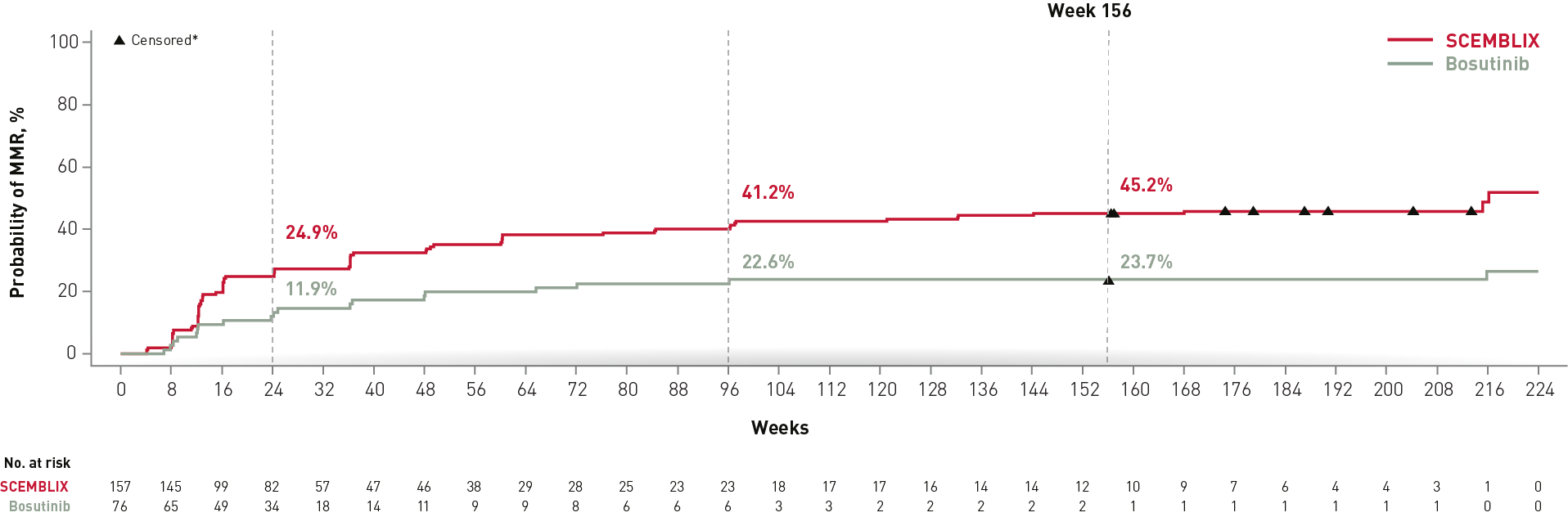
*Non-responders were censored at their last molecular assessment date.
Adapted from Réa D, et al. 2021,2 Mauro M, et al. 20234 and Mauro M, et al. 2023.8
SCEMBLIX achieved higher MMR rates vs bosutinib throughout the duration of the study.2,4
SCEMBLIX resulted in fewer all-grade and grade ≥3 AEs vs bosutinib2,3
AE, adverse event; ATP, adenosine triphosphate; BCR-ABL, breakpoint cluster region and Abelson murine leukaemia viral oncogene homologue; CCyR, complete cytogenetic remission; CI, confidence interval; CML-CP, chronic myeloid leukaemia in chronic phase; IS, international scale; MCyR, major cytogenetic response; MMR, major molecular response; MR, molecular response; Ph+, Philadelphia chromosome positive; STAMP, specifically targeting the ABL1 myristoyl pocket; TKI, tyrosine kinase inhibitor.
For further information, please refer to the Summary of Product Characteristics.
References
SCEMBLIX (asciminib) Summary of Product Characteristics.
Réa D, et al. Blood 2021;138(21):2031–2041 (and supplementary appendix).
Hochhaus A, et al. Leukemia 2023;37:617–626 (and supplementary appendix).
Mauro M, et al. Blood 2023;142 (Supplement 1):4536–4539.
Hehlmann R, et al. Leukemia 2017;31(11):2398–2406.
Castagnetti F, et al. Leukemia 2015;29(9):1823–1831.
Jabbour E, et al. Blood 2011;118(17):4541–4546.
Mauro M, et al. Poster 4536. 65th ASH Annual Meeting & Exposition; December 9–12, 2023; San Diego, California, & virtual.
UK | November 2024 | FA-11311637
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.

