Cosentyx patient resources
Useful resources to support you in your day-to-day practice.
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
Cosentyx is indicated for the treatment of: moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adults (alone or in combination with methotrexate [MTX]) who have responded inadequately to disease-modifying anti-rheumatic drug therapy; active ankylosing spondylitis (AS) in adults who have responded inadequately to conventional therapy; active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation as indicated by elevated C-reactive protein and/or magnetic resonance imaging evidence in adults who have responded inadequately to non-steroidal anti-inflammatory drugs; active enthesitis-related arthritis (ERA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate conventional therapy; active juvenile psoriatic arthritis (JPsA) in patients 6 years or older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.1
Please refer to the Summary of Product Characteristics (SmPC) for further information.
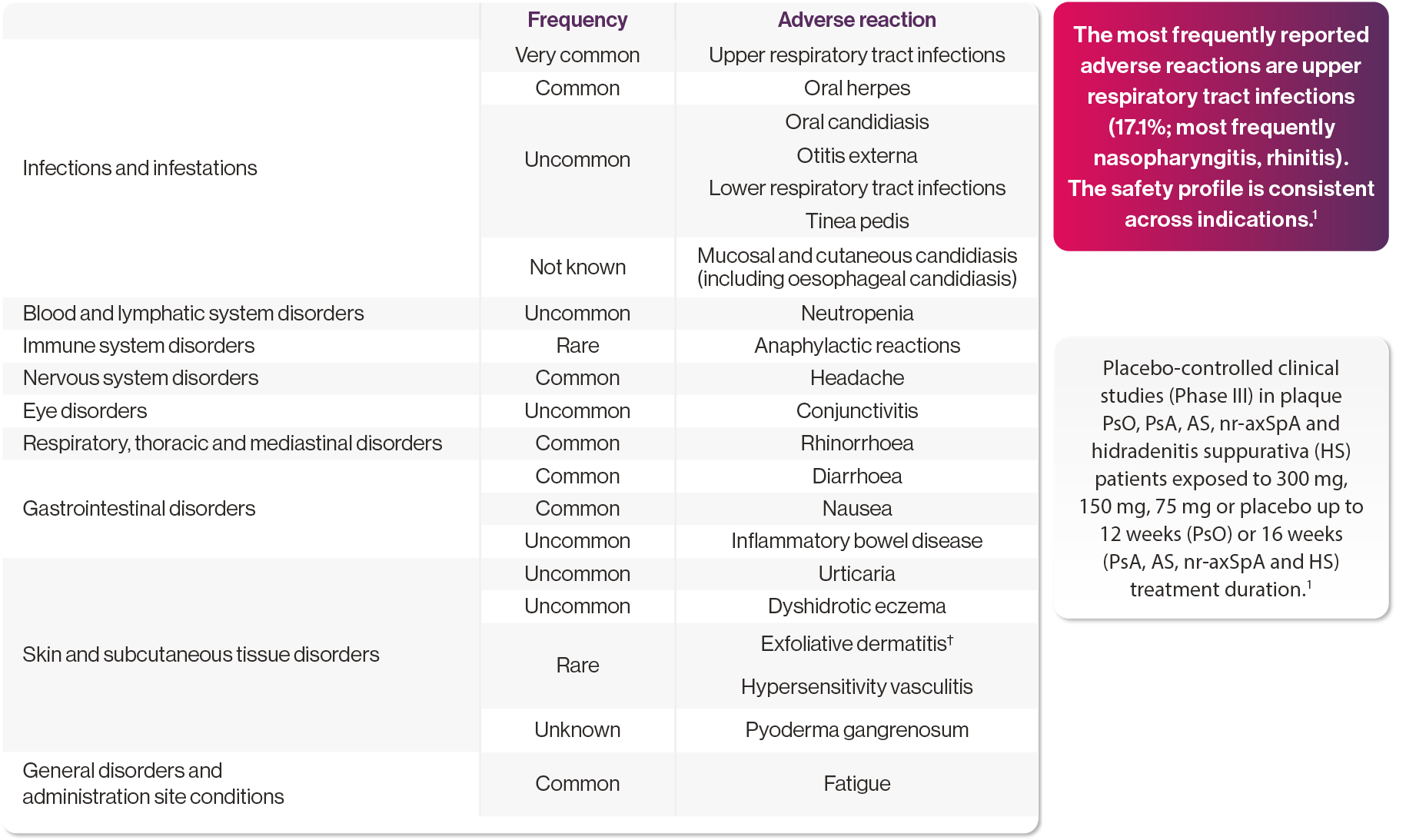
No new safety signals in clinical trials, including those for paediatric patients with PsO (n=162) and juvenile idiopathic arthritis (JIA) (n=86) as young as 6 years of age1
No trend towards increased rates of major adverse cardiovascular event (MACE) or malignancy reported in clinical trials or RWE*6
No discontinuations in pooled clinical trial data and RWE due to Candida infections, with all being non-serious and mild to moderate in severity, except four cases in the PsO pool that were considered severe6
<1% of patients with PsO had immunogenicity over 5 years7
No lab monitoring required for neutropenia, lymphopenia, anaemia, lipids1
No warning in SmPC about use in patients with a history of smoking1
Adapted from Cosentyx SmPC. Please refer to the SmPC for full information on adverse events.
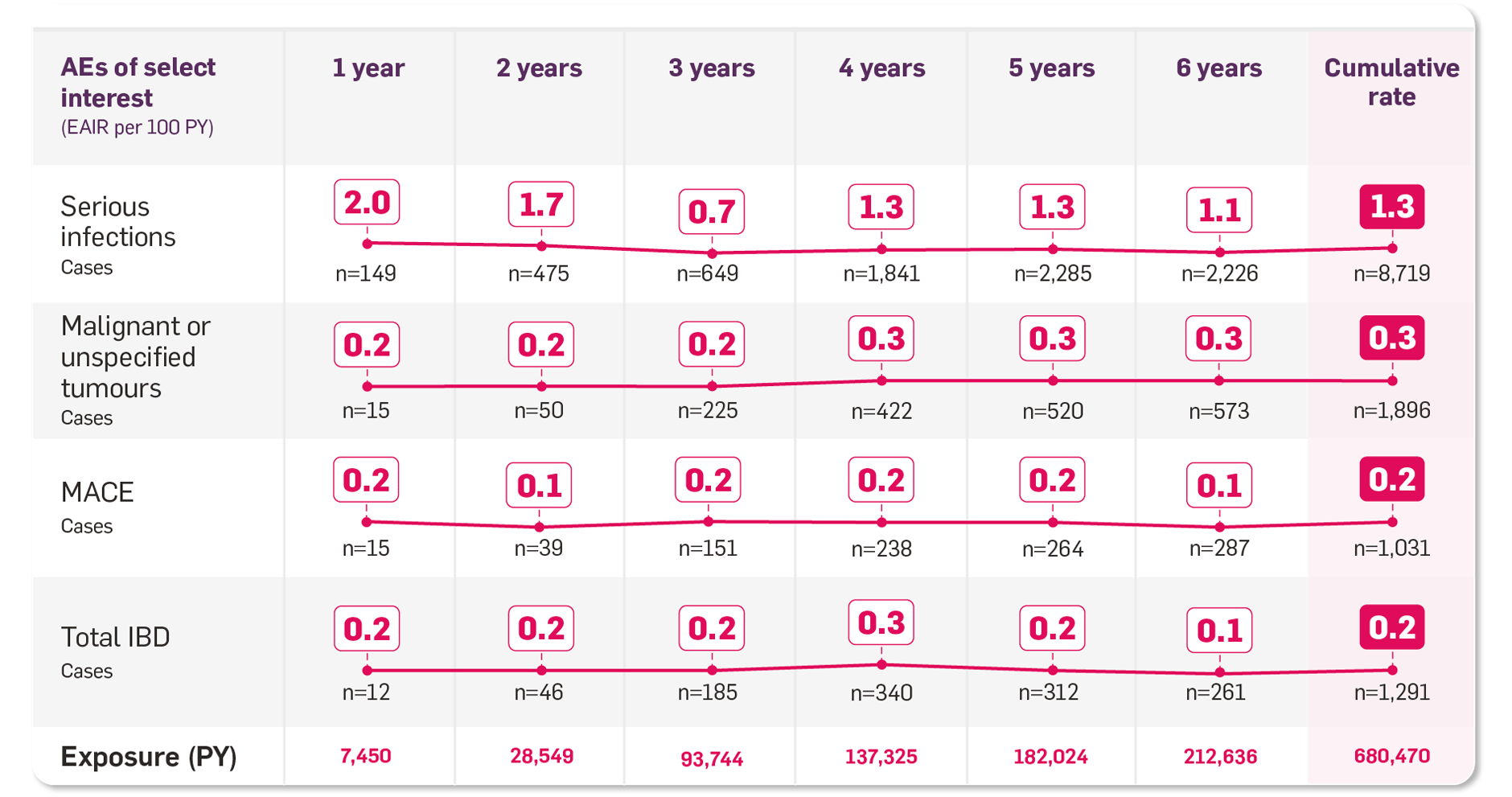
No trend toward increased adverse event (AE) rates over time (pooled AS, PsA, plaque PsO in a Periodic Safety Update Report [PSUR], including exposure in clinical trials and marketing experience)8
Adapted from Cosentyx PSUR 2021.8
Therapeutic Indications1
Cosentyx is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adult patients (alone or in combination with methotrexate [MTX]) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active ankylosing spondylitis (AS) in adults who have responded inadequately to conventional therapy; active nonradiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation as indicated by elevated C-reactive protein and/or magnetic resonance imaging evidence in adults who have responded inadequately to non-steroidal anti-inflammatory drugs; active moderate to severe hidradenitis suppurativa (HS; acne inversa) in adults with an inadequate response to conventional systemic HS therapy; active enthesitis-related arthritis (ERA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active juvenile psoriatic arthritis (JPsA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.1
*The pooled clinical trial safety data for Cosentyx involved 7, 300+ patients and 21 randomised controlled clinical trials, including long-term exposure of up to 5 years in PsO and PsA and up to 4 years in AS. The post-marketing data are from the Cosentyx PSUR submitted to global health authorities.6
†Cases were reported in patients with PsO diagnosis.1
‡Successive time periods of PSUR shown with cumulative rate: 26 Dec 2014 to 25 Dec 2015; 26 Dec 2015 to 25 Dec 2016; 26 Dec 2016 to 25 Dec 2017; 26 Dec 2017 to 25 Dec 2018; 26 Dec 2018 to 25 Dec 2019; 26 Dec 2019 to 25 Dec 2020.8
AE, adverse event; AS, ankylosing spondylitis; axSpA, axial spondyloarthritis; EAIR, exposure-adjusted incidence rate; ERA, enthesitis-related arthritis; HS, hidradenitis suppurativa; IBD, inflammatory bowel disease; JIA, juvenile idiopathic arthritis; JPsA, juvenile psoriatic arthritis; MACE, major adverse cardiovascular event; MTX, methotrexate; nr-axSpA, non-radiographic axial spondyloarthritis; PsA, psoriatic arthritis; PsO, psoriasis; PSUR, periodic safety update report; PY, patient-years; RWE, real world evidence; SmPC, summary of product characteristics.
References
Cosentyx® (secukinumab) Summary of Product Characteristics.
Baraliakos X, et al. RMD Open 2019;5(2):e001005.
Marzo-Ortega H, et al. Ann Rheum Dis 2019;78(suppl 2):873. Abstract FRI0379.
Mease PJ, et al. ACR Open Rheumatol 2020;2(1):18–25.
McInnes IB, et al. Lancet Rheumatol 2020;2(4):e227–e235.
Deodhar A, et al. Arthritis Res Ther 2019;21(1):111.
Reich K, et al. J Eur Acad Dermatol Venereol 2019;33(9):1733–1741.
Novartis Data on File. Cosentyx PSUR; 26 December 2019–25 December 2020. 22 February 2021.
UK | January 2025 | FA-11328448
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.