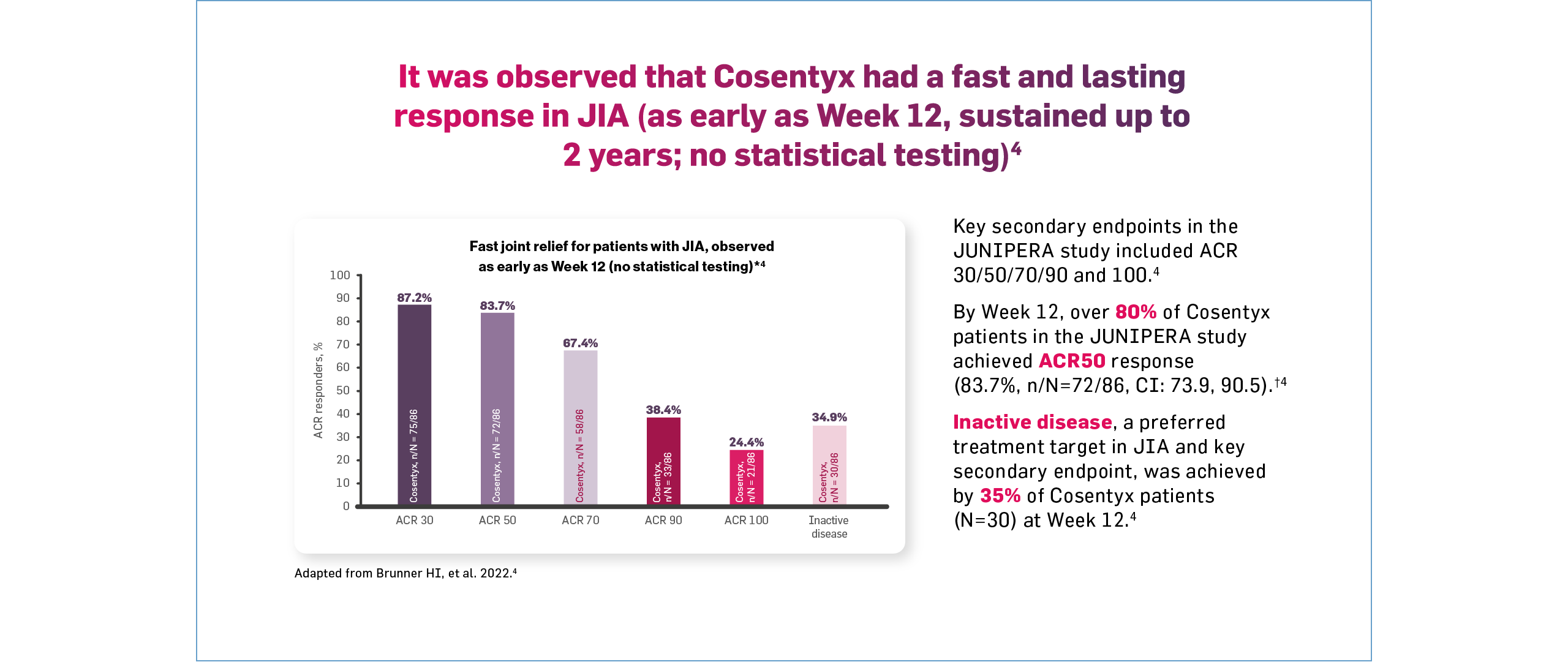
†In Treatment Period 1, all patients received Cosentyx until Week 12. Key secondary endpoints included ACR 30/50/70/90 and 100. At Week 1, 33.7%, 22.1%, 8.1%, 2.3% and 1.2% of children with JIA were JIA ACR 30, 50, 70, 90 and 100 responders, respectively.4 At Week 12, 87%, 84%, 67%, 38% and 24% of children with JIA were JIA-ACR 30, 50, 70, 90 and 100 responders, respectively. A total of 34.9% of patients with JIA reached inactive disease status at Week 12.4
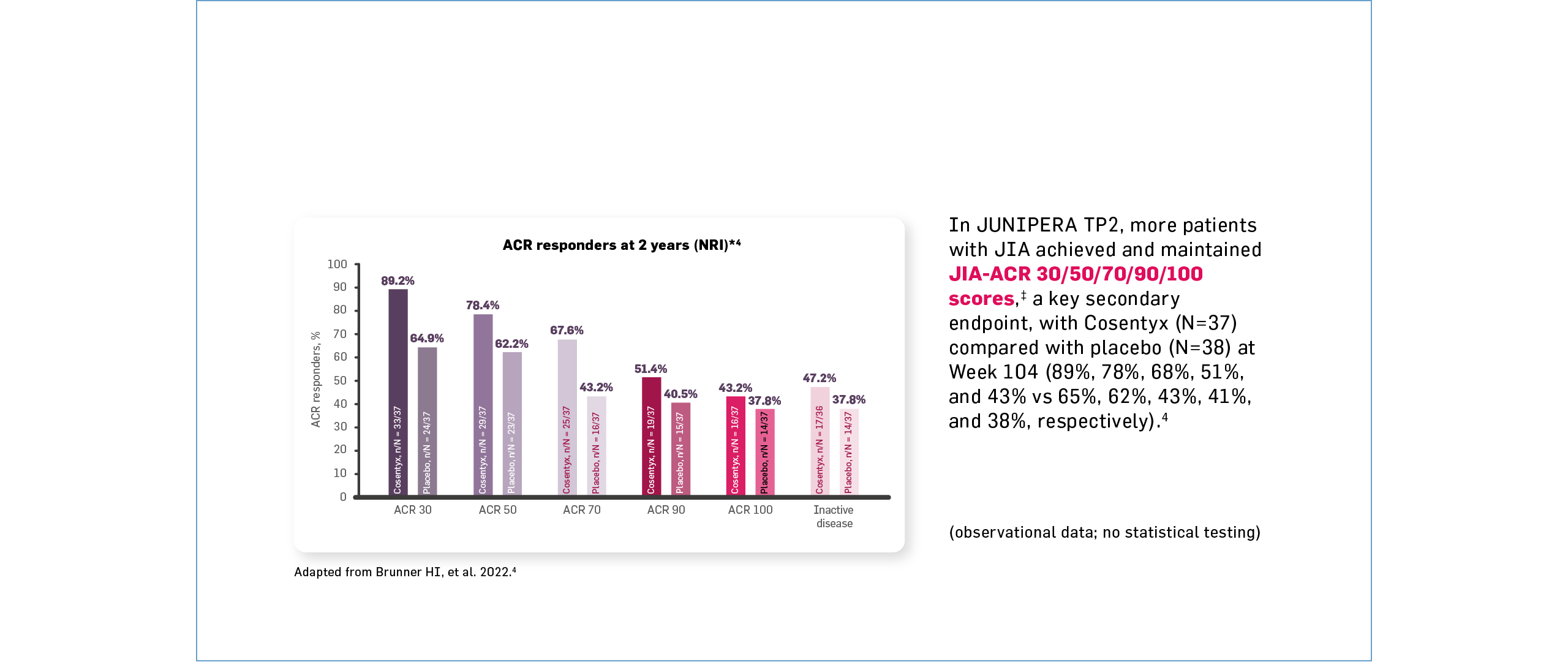
‡The JIA ACR30/50/70/90/100 response as per the JIA-ACR response criteria is defined as 30/50/70/90/100% improvement in three or more of six CRVs, with no more than one of the remaining CRVs worsening by >30%.1
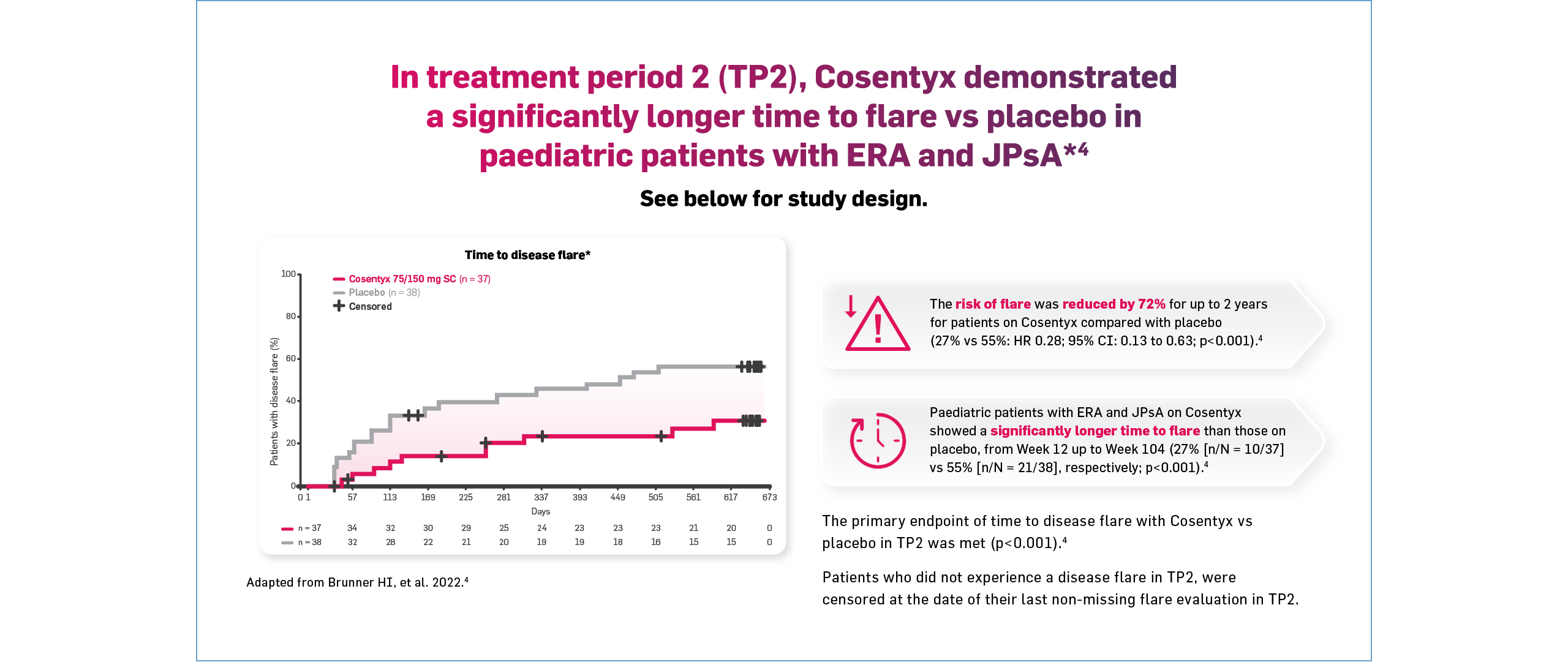
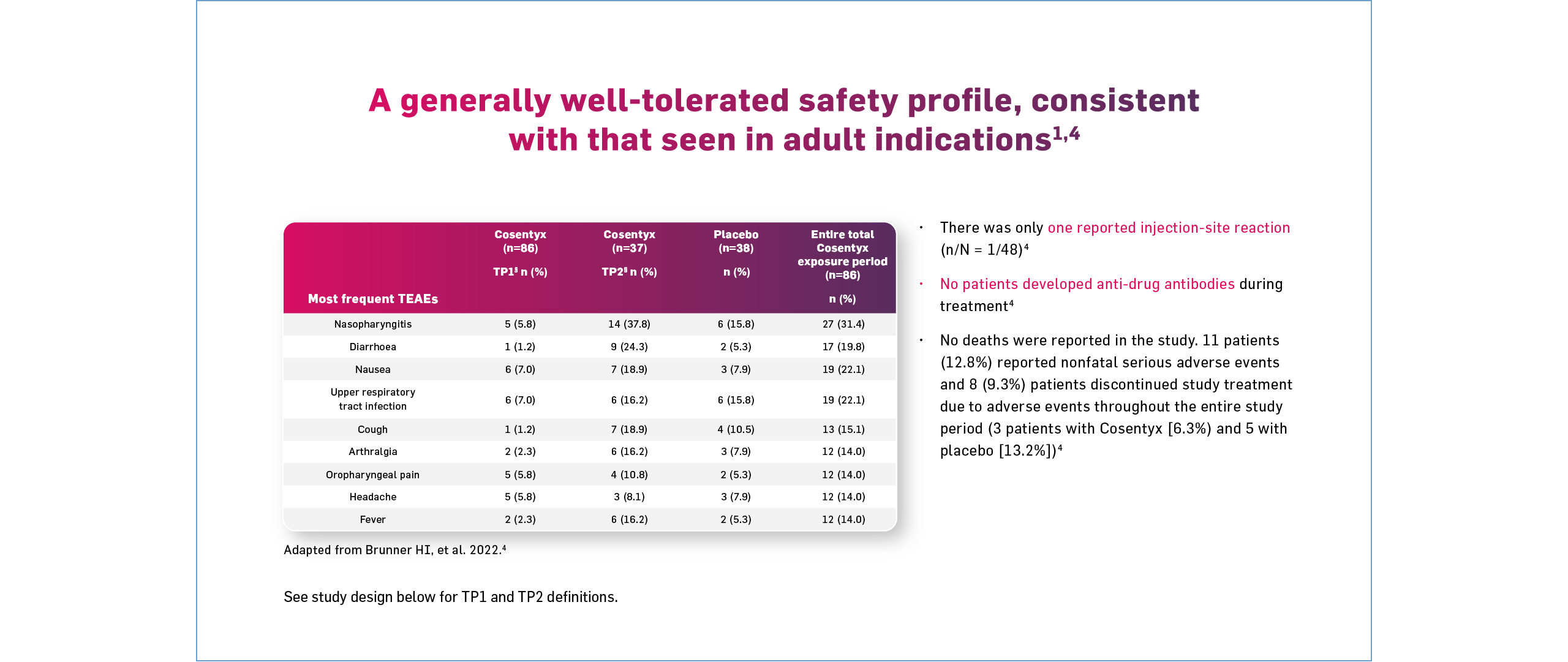
§Disease flare was defined as ≥30% worsening from baseline in ≥3 of the 6 JIA ACR response criteria, >30% improvement relative to the end of Week 12.4
ACR, American College of Rheumatology; AS, ankylosing spondylitis; axSpA, axial spondyloarthritis; CI, confidence interval; CRP, C-reactive protein; CRV, core set variable; DMARD, disease modifying anti-rheumatic drug; ERA, enthesitis-related arthritis; HR, hazard ratio; IL-17A, interleukin 17A; JADAS, juvenile arthritis disease activity score; JIA, juvenile idiopathic arthritis; JPsA, juvenile psoriatic arthritis; MoA, mechanism of action; MRI, magnetic resonance imaging; MTX, methotrexate; nr-axSpA, non-radiographic axial spondyloarthritis; NSAID, non-steroidal anti-inflammatory drug; PsA, psoriatic arthritis; PsO, plaque psoriasis; TP2, Treatment Period 2.
References
Cosentyx® (secukinumab) Summary of Product Characteristics.
Taltz (ixekizumab) Summary of Product Characteristics.
Bimzelx (bimekizumab) Summary of Product Characteristics.
Brunner HI, et al. Ann Rheum Dis 2022;82:154–160.
Paroli M, et al. Medicina 2022;58:1552.
Tsukazaki H, et al. Int J Mol Sci 2020;21:6401.
Novartis Data on File. CAIN457F2304. Data Analysis Report. January 2022.