Contact us
Our dedicated team of Novartis representatives based throughout the UK are on hand to answer your local query.
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
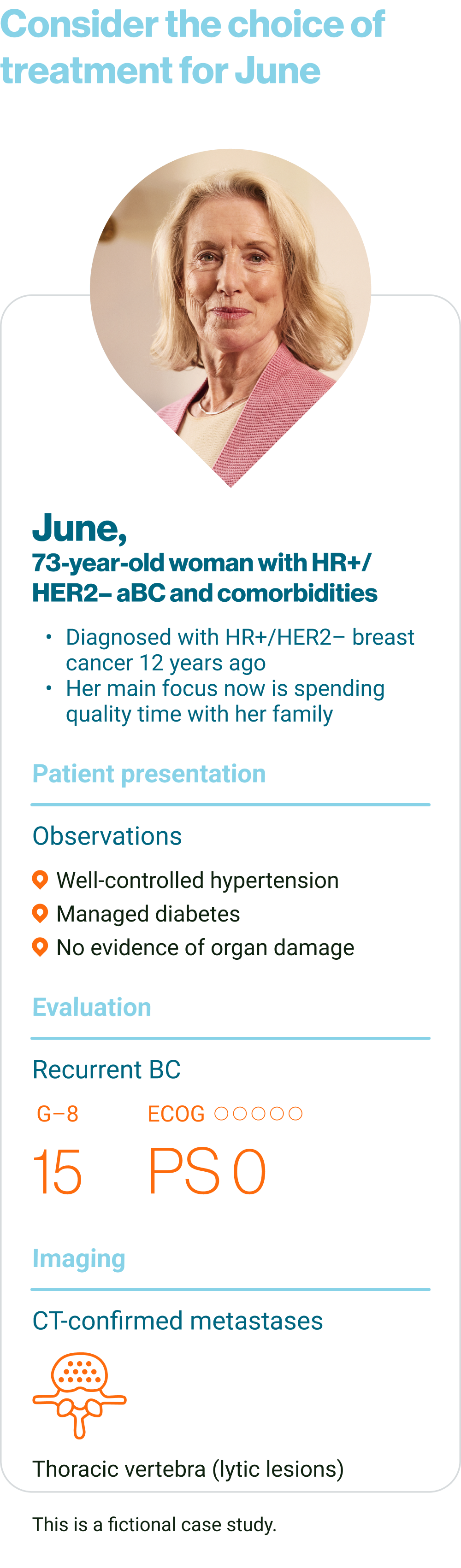
KISQALI is indicated for the treatment of women with hormone receptor (HR)-positive human epidermal growth factor receptor 2 (HER2)-negative locally advanced or metastatic breast cancer in combination with an aromatase inhibitor or fulvestrant as initial endocrine-based therapy or in women who have received prior endocrine therapy
In pre- or perimenopausal women the endocrine therapy should be combined with a luteinising hormone-releasing hormone (LHRH) agonist
KISQALI is not recommended to be used in combination with tamoxifen.
For more safety information, click here.
For the full safety profile, please refer to the KISQALI Summary of Product Characteristics.
Clinical responses vary; please refer to the Summary of Product Characteristics prior to prescribing. No statistically significant differences in mOS were found in observational studies with patients >65 years treated with KISQALI compared to placebo.
MONALEESA-2, -3, and -7 exploratory pooled analysis
Median overall survival in patients <65 years:
Adapted from Hart L, et al. 2023.1
This was an exploratory analysis of pooled MONALEESA data to assess PFS, OS and TTC across age groups (<65, 65–74 and ≥75 years) using Kaplan-Meier methods.¹
Exploratory subgroup analyses are signal seeking and hypothesis generating; event numbers and sample sizes are limited.
ET was defined as either AI or fulvestrant.
MONALEESA-2, -3, and -7 exploratory pooled analysis
Median overall survival in patients 65–74 years:
Adapted from Hart L, et al. 2023.1
This was an exploratory analysis of pooled MONALEESA data to assess PFS, OS and TTC across age groups (<65, 65–74 and ≥75 years) using Kaplan-Meier methods.1
Exploratory subgroup analyses are signal seeking and hypothesis generating; event numbers and sample sizes are limited.
ET was defined as either AI or fulvestrant.
MONALEESA-2, -3, and -7 exploratory pooled analysis
Median overall survival in patients ≥75 years:*
Adapted from Hart L, et al. 2023.1
This was an exploratory analysis of pooled MONALEESA data to assess PFS, OS and TTC across age groups (<65, 65–74 and ≥75 years) using Kaplan-Meier methods.1
Exploratory subgroup analyses are signal seeking and hypothesis generating; event numbers and sample sizes are limited.
ET was defined as either AI or fulvestrant.
*The ≥75-year age group has a small sample size (n=121), and data should be interpreted with caution.1
MONALEESA-2, -3, and -7 exploratory pooled analysis
Of all patients who received KISQALI in studies MONALEESA-2 and MONALEESA-3, representative proportions of patients were ≥65 years and ≥75 years of age. No overall differences in safety or effectiveness of KISQALI were observed between these patients.2
Study designs
MONALEESA-2: N=668, double-blind, placebo-controlled, 1:1 randomised, multicentre, Phase III trial in postmenopausal women with HR+/HER2− aBC. As 1L in advanced disease. No prior ET for aBC and no previous systemic chemotherapy for advanced disease. KISQALI 600 mg or placebo orally once daily (3 weeks on/1 week off) + AI (2.5 mg continuous). The primary endpoint was locally assessed PFS and the key secondary endpoint was OS. Other secondary endpoints included the ORR (complete or partial response), CBR (overall response plus stable disease lasting 24 weeks or more), safety, and QoL assessments.3
MONALEESA-3: N=726, double-blind, placebo-controlled, 2:1 randomised, Phase III trial. As 1L and 2L in advanced disease plus those with early relapse in postmenopausal women with HR+/HER2– aBC. KISQALI 600 mg or placebo orally once daily (3 weeks on/1 week off) + 500 mg intramuscular fulvestrant. The primary endpoint was locally assessed PFS. Secondary endpoints included OS, ORR, CBR, and safety and tolerability. No prior ET or after disease progression on 1L ET for aBC.4
MONALEESA-7: N=672, double-blind, placebo-controlled, 1:1 randomised, Phase III trial in pre/perimenopausal women with HR+/HER2− aBC. As 1L in advanced disease and in patients who received 1 or fewer lines of chemotherapy for aBC. KISQALI 600 mg or placebo orally once daily (3 weeks on/1 week off) + NSAI (letrozole 2.5 mg or anastrozole 1 mg) or tamoxifen 20 mg orally once daily continuously + LHRH agonist (goserelin 3.6 mg subcutaneously on Day 1 of every cycle). The primary endpoint was investigator assessed PFS. The key secondary endpoint was OS, defined as the time from randomisation to death from any cause. The other secondary endpoints included: proportion of patients who achieved an objective response, clinical benefit, TTR, duration of response, time to definitive deterioration of ECOG PS from baseline, time to 10% deterioration for EORTC QLQ-C30 and safety.5
KISQALI is not recommended to be used in combination with tamoxifen.2
1L, first-line; 2L, second-line; aBC, advanced breast cancer; AE, adverse event; AI, aromatase inhibitor; BC, breast cancer; CBR, clinical benefit rate; CI, confidence interval; CT, computed tomography; ECOG, Eastern Cooperative Oncology Group performance status; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer core quality of life questionnaire; ET, endocrine therapy; HER2–, human epidermal growth factor receptor 2 negative; HR, hormone receptor; LHRH, luteinising hormone-releasing hormone; mOS, median overall survival; NSAI, non-steroidal aromatase inhibitor; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; QoL, quality of life; TTR, time to response.
References
Hart L, et al. Poster presentation PS02-01. Presented at San Antonio Breast Cancer Symposium 2023, 5–9 December, San Antonio, USA.
KISQALI (ribociclib) Summary of Product Characteristics.
Hortobagyi GN, et al. N Engl J Med 2016;375(18):1738–1748.
Slamon DJ, et al. J Clin Oncol 2018;36(24):2465–2472.
Tripathy D, et al. Lancet Oncol 2018 Jul;19(7):904–915.
UK | April 2025 | FA-11323500-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.