Contact us
Our dedicated team of Novartis representatives based throughout the UK are on hand to answer your local query.
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
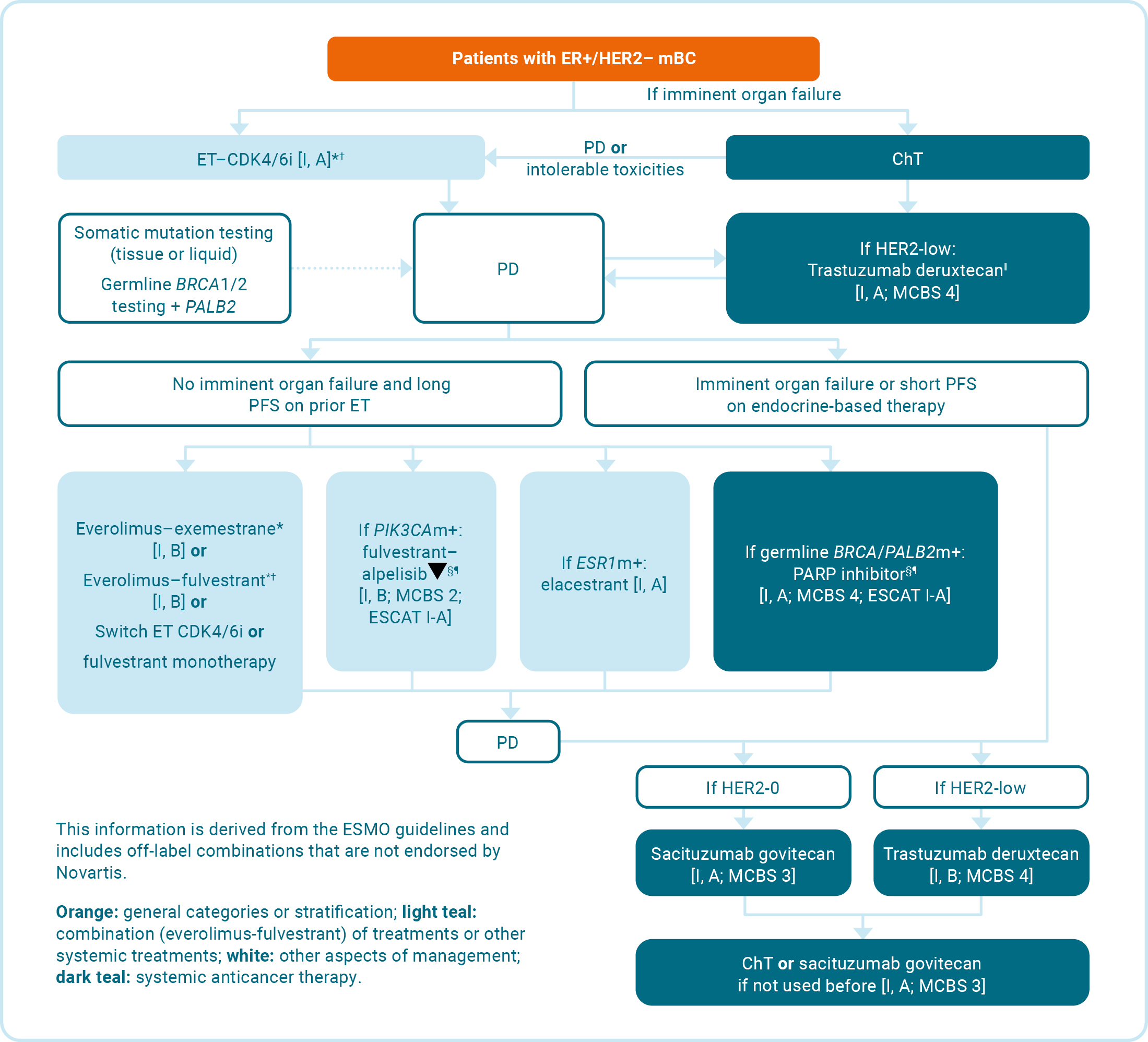
KISQALI is indicated for the treatment of women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative locally advanced or metastatic breast cancer in combination with an aromatase inhibitor or fulvestrant as initial endocrine-based therapy, or in women who have received prior endocrine therapy
In pre- or perimenopausal women, the endocrine therapy should be combined with a luteinising hormone-releasing hormone agonist
KISQALI is not recommended to be used in combination with tamoxifen.
Treatment is aimed at improving OS, in addition to maintaining or improving quality of life.3 Although the disease remains incurable, the availability of systemic therapies such as endocrine therapy (ET) has led to improvements in the management of this hormone-sensitive disease.4
Cyclin-dependent kinases 4 and 6 (CDK4/6) play a key role in cell cycle progression and have become an effective target in the treatment of aBC.5
KISQALI is not recommended to be used in combination with tamoxifen.1
Adapted from European Society for Medical Oncology. 2023.6
Everolimus is not licensed in combination with fulvestrant or for use in premenopausal women. Novartis does not condone the off-label use of therapies. Please refer to individual Summary of Product Characteristics before prescribing.
Alpelisib is indicated in combination with fulvestrant for the treatment of postmenopausal women, and men, with HR+/HER2–, locally advanced or mBC with a PIK3CA mutation after disease progression following endocrine-based therapy.7 Please consult the Summary of Product Characteristics before prescribing.
ET is defined as NSAI or tamoxifen and LHRH.
The ESMO-MCBS is a standardised tool that quantifies the likely magnitude of clinical benefit. The scale considers OS, PFS, disease-free survival, hazard ratio, response rate, quality of life, prognosis of the condition and toxicity of patients in the non-curative setting, ranging from grades 1 to 5, with 4 and 5 denoting substantial clinical benefit.9
For further information, please refer to the ESMO-MCBS scorecard methodology.9
*OFS if the patient is premenopausal.6
†If relapse <12 months after end of adjuvant AI: fulvestrant–CDK4/6i;* if relapse >12 months after end of adjuvant AI: AI–CDK4/6i.*6
‡Preferred if the patient is ESR1m+ [ESCAT score: II-A].§6
§ESMO-MCBS v1.1 was used to calculate scores for therapies/indications approved in the EU and US. The scores have been calculated by the ESMO-MCBS Working Group and validated by the ESMO Guidelines Committee.10
¶ESCAT scores apply to genomic alterations only. These scores have been defined by the guideline authors and validated by the ESMO Translational Research and Precision Medicine Working Group.11
‖Trastuzumab deruxtecan can also be given following adjuvant ChT in the setting of fast progression (DESTINY-Breast04).6
aBC, advanced breast cancer; AI, aromatase inhibitor; BRCA, breast cancer gene; CDK4/6i, cyclin-dependent kinase 4/6-inhibitor; ChT, chemotherapy; ER, oestrogen receptor; ESCAT, ESMO scale for clinical actionability of molecular targets; ESMO, European Society for Medical Oncology; ESMO-MCBS, European Society for Medical Oncology magnitude of clinical benefit scale; ESR1, oestrogen receptor 1; ET, endocrine therapy; HER2–, human epidermal growth receptor 2-negative; HR+, hormone receptor-positive; m, mutated; mBC, metastatic breast cancer; OFS, ovarian function suppression; PALB2, partner and localiser of BRCA2; PARP, poly ADP-ribose polymerase; PD, progressive disease; PFS, progression-free survival; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha.
References:
KISQALI® (ribociclib) Summary of Product Characteristics.
Cardoso F, et al. Ann Oncol 2020;31(12):1623–1649.
Hortobagyi GN, et al. N Engl J Med 2022;386:942–950.
McAndrew NP, et al. JCO Oncol Prac 2021;18(5):319–327.
Slamon DJ, et al. Annals Oncol 2021;32(8):1015–1024.
European Society for Medical Oncology. ER-positive HER2-negative Breast Cancer, v1.1 May 2023. Available at: https://www.esmo.org/living-guidelines/esmo-metastatic-breast-cancer-living-guideline/er-positive-her2-negative-breast-cancer [Accessed March 2025].
PIQRAY® (alpelisib) Summary of Product Characteristics.
European Society for Medical Oncology. Breast cancer. Available at: https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-for-solid-tumours/esmo-mcbs-scorecards?cbs_score_cards_form%5BsearchText%5D=&mcbs_score_cards_form%5Btumourtype%5D=0&mcbs_score_cards_form%5Btumour-sub-type%5D=Breast+Cancer [Accessed March 2025].
European Society for Medical Oncology. ESMO-magnitude of clinical benefit scale. Available at: https://www.esmo.org/content/download/288502/5736211/1/esmo-mcbs-booklet.pdf [Accessed March 2025].
Cherny NI, et al. Ann Oncol 2017;28(10):2340–2366.
Mateo J, et al. Ann Oncol 2018;29(9):1895–1902.
UK | March 2025 | 442231
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.