Please refer to the Cosentyx SmPC for full product information and administration, including dosing in special populations, before prescribing.1
Therapeutic indications1
Cosentyx is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adult patients (alone or in combination with methotrexate [MTX]) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active ankylosing spondylitis (AS) in adults who have responded inadequately to conventional therapy; active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation as indicated by elevated C-reactive protein and/or magnetic resonance imaging evidence in adults who have responded inadequately to non-steroidal anti-inflammatory drugs; active moderate to severe hidradenitis suppurativa (HS; acne inversa) in adults with an inadequate response to conventional systemic HS therapy; active enthesitis-related arthritis (ERA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active juvenile psoriatic arthritis (JPsA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.
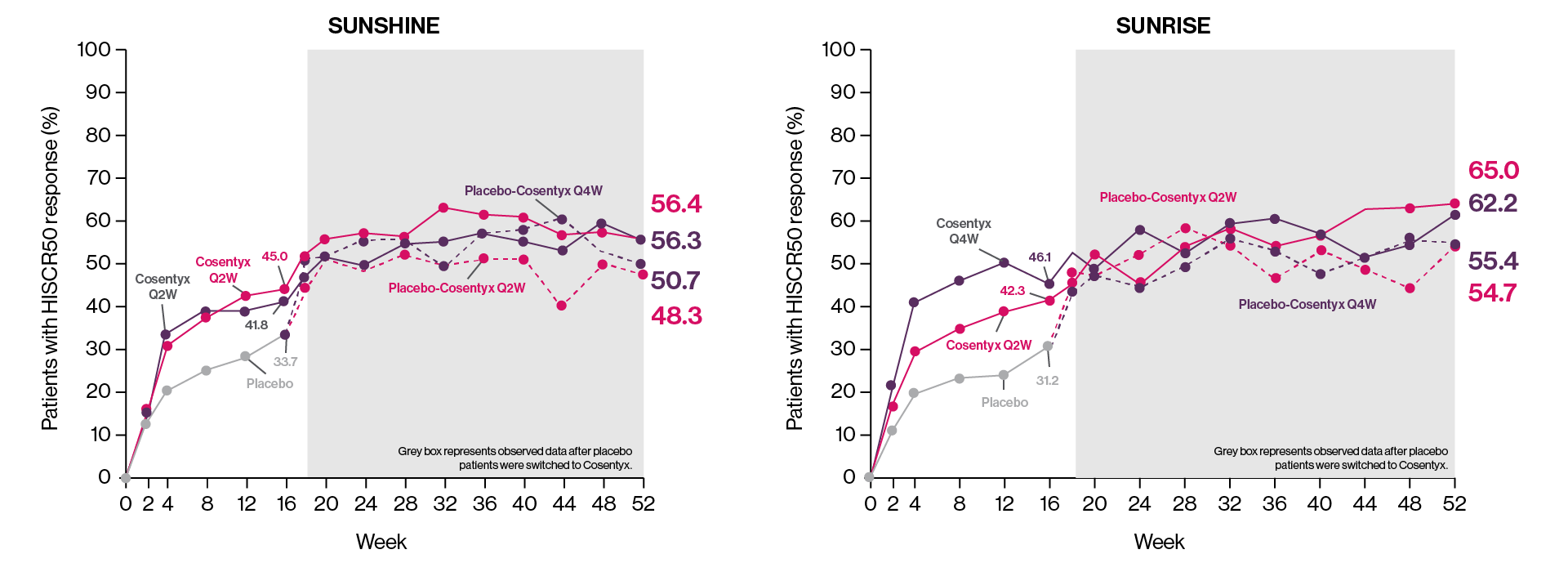
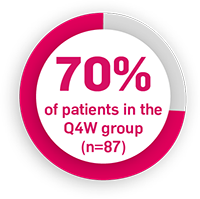
AN50, decrease in abscess and inflammatory nodule count by 50%; AS, ankylosing spondylitis; CFB, change from baseline; ERA, enthesitis-related arthritis; HiSCR50, hidradenitis suppurativa clinical response 50% reduction from baseline; HS, hidradenitis suppurativa; JPsA, juvenile psoriatic arthritis; MTX, methotrexate; nr-axSpA, non-radiographic axial spondyloarthritis; NRS, numeric rating scale; PsA, psoriatic arthritis; PsO, plaque psoriasis; Q2W, every 2 weeks; Q4W, every 4 weeks; SmPC, summary of product characteristics.
References
Cosentyx® (secukinumab) Summary of Product Characteristics.
Kimball A, et al. Lancet 2023;401(10378):747–761; and supplementary appendix.
Vinkel C & Thomsen SF. J Clin Aesthet Dermatol 2018;11(10):17–23.
Kimball A, et al. Poster presented at the American Academy of Dermatology (AAD) 2023, March 17–21, New Orleans, USA.
Novartis Data on File. SUNNY Clinical Study Program post-hoc analysis of skin pain severity.
Bechara FG, et al. P144. Poster presented at the European Hidradenitis Suppurativa Foundation e.V. (EHSF); February 8–10, 2023; Florence, Italy.