

LEQVIO®▼ (inclisiran) may help your patients reach LDL-C targets with 2 maintenance doses a year in combination with a maximally tolerated statin1
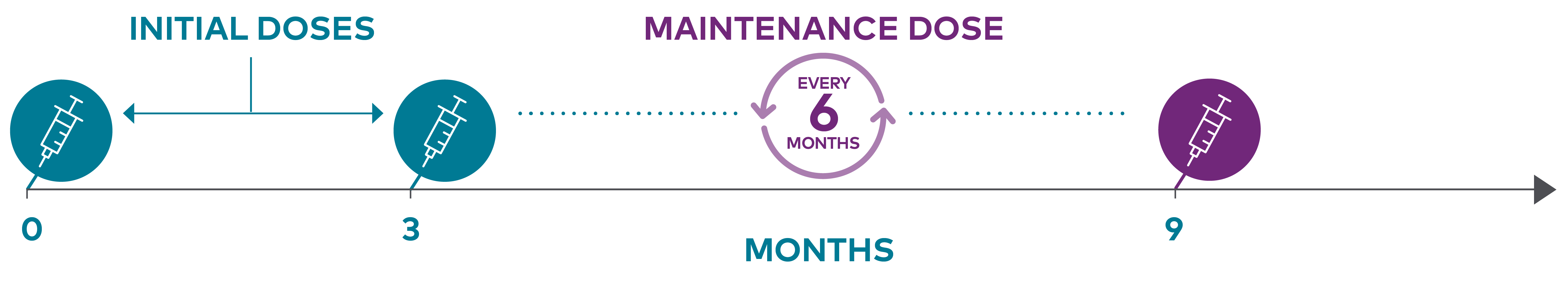
After an initial dose, LEQVIO® is administered again at 3 months, followed by every 6 months1
LEQVIO® is indicated in adults with primary hypercholesterolaemia (heterozygous familial and non-familial) or mixed dyslipidaemia, as an adjunct to diet:1
in combination with a statin or statin with other lipid-lowering therapies in patients unable to reach LDL-C goals with the maximum tolerated dose of a statin, or
alone or in combination with other lipid-lowering therapies in patients who are statin-intolerant, or for whom a statin is contraindicated.
For full safety information, please refer to the LEQVIO® Summary of Product Characteristics.
LEQVIO® dosing and administration
LEQVIO® is the first and only siRNA LDL-C-lowering therapy,1–3 with the convenience of
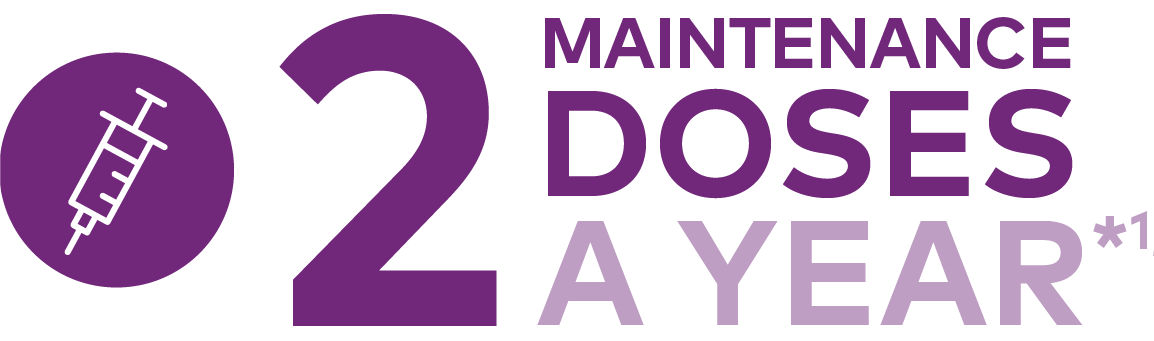
HCP-administered
Subcutaneous injection
No refrigeration required
Fixed dose
No additional monitoring blood tests mandated†
Does not add to pill burden
*After an initial dose, LEQVIO® is administered again at 3 months, followed by every 6 months.1
†Beyond what is already clinically indicated for your patient.
No data are available in patients with severe hepatic impairment (Child-Pugh class C). LEQVIO® should be used with caution in patients with severe hepatic impairment.
There is limited experience with LEQVIO® in patients with severe renal impairment. LEQVIO® should be used with caution in these patients.
The effect of haemodialysis on LEQVIO® pharmacokinetics has not been studied. Considering that LEQVIO® is eliminated renally, haemodialysis should not be performed for at least 72 hours after LEQVIO® dosing.
Watch the short video below to learn more about LEQVIO® administration, including a step-by-step guide for injection.
Subcutaneous administration of LEQVIO®:1
The recommended dose of LEQVIO® is 284 mg, administered via a single pre-filled syringe: initially, again at 3 months, and thereafter every 6 months.
For full information on LEQVIO® dosing and administration, please refer to the SmPC.
Additional dosing and administration information1

No dose adjustments are required for patients with mild or moderate hepatic impairment, mild, moderate or severe renal impairment or end-stage renal disease, or elderly patients (age ≥65 years).

Check the LEQVIO® solution for injection visually before administration – it should be clear, colourless to pale yellow and essentially free of particles. If the solution contains visible particulate matter, the solution should not be used. Any unused medicinal product or waste material should be disposed of in accordance with local requirements.

Areas to avoid: active skin disease or injury (e.g. sunburns, skin rashes, inflammation, skin infections).

If a planned dose is missed by less than 3 months, LEQVIO® should be administered and dosing continued according to the patient's original schedule. If a planned dose is missed by more than 3 months, a new dosing schedule should be started – LEQVIO® should be administered initially, again at 3 months, followed by every 6 months.

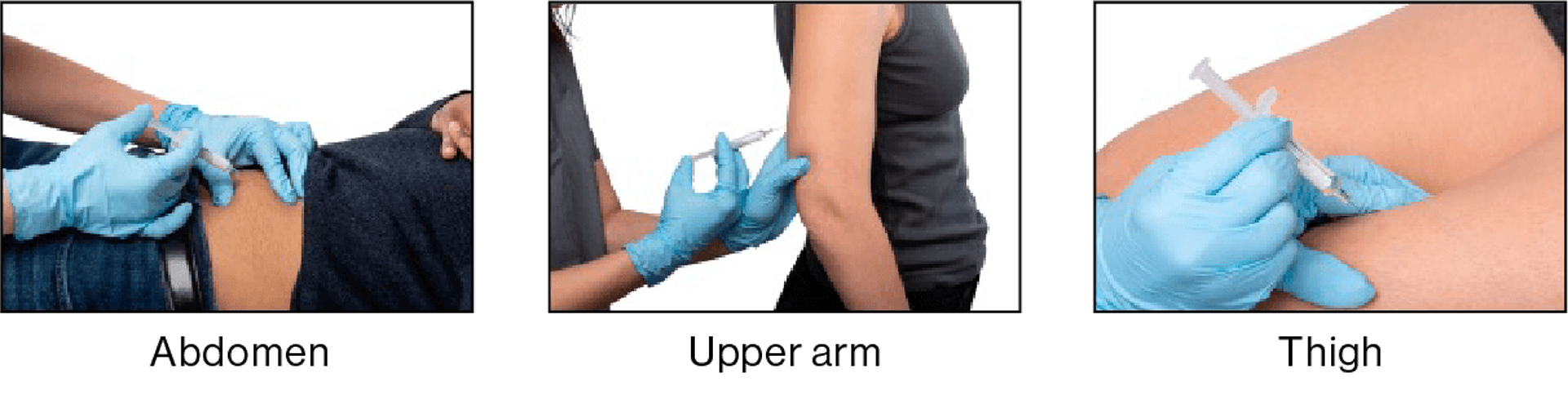
LEQVIO® is administered by a healthcare professional into the abdomen, upper arm or thigh.
For further information, please refer to the SmPC.
With over 12,000 patient-years exposure and >20,000 injections, the safety profile of LEQVIO® remains consistent, with no new safety signals identified in the ORION-8 open-label extension study‡4
With straightforward twice-yearly maintenance dosing, LEQVIO® can be administered during existing patient appointments1
After an initial dose, LEQVIO® is administered again at 3 months, followed by every 6 months.1
Explore LEQVIO® eligibility criteria
Explore LEQVIO® eligibility criteria
‡A pooled cohort of 3274 patients treated with LEQVIO® with an assumed dosing frequency of two injections per year and an average treatment duration of 3.7 years.4
HCP, healthcare professional; LDL-C, low-density lipoprotein cholesterol; siRNA, small interfering ribonucleic acid; SmPC, summary of product characteristics.
References
LEQVIO® Summary of Product Characteristics.
Stoekenbroek RM, et al. Future Cardiol 2018;14(6):433–442.
Klinovski M, et al. Inclisiran: a small interfering RNA molecule for treating hypercholesterolemia. Ottawa: CADTH; 2019. CADTH Issues in Emerging Health Technologies; Issue 180.
Wright RS, et al. Oral presentation. ORION-8: Long-term efficacy and safety of twice-yearly inclisiran in high cardiovascular risk patients. ESC Congress 2023. Amsterdam, The Netherlands, 25–28 August 2023.
LEQVIO® and the LEQVIO® logo are registered trademarks of Novartis AG. Licensed from Alnylam Pharmaceuticals, Inc.
UK | January 2025 | FA-11205606
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.


