

Cosentyx® (secukinumab): Safety profile and side effects
Cosentyx® (secukinumab) is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adult patients (alone or in combination with methotrexate [MTX]) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active moderate to severe hidradenitis suppurativa (HS; acne inversa) in adults with an inadequate response to conventional systemic HS therapy.1
Please refer to the Summary of Product Characteristics (SmPC) for further information. The UK SmPC can be found here.
Cosentyx demonstrates a consistent safety profile across licensed indications supported by up to 8 years of data1,2
<3% Candida infections over 5 years3
<1% immunogenicity at 52 weeks1
No new safety signals in clinical trials, including those for paediatric patients with PsO (n=162) as young as 6 years of age1
Comparable safety profile in both Q2W and Q4W maintenance dosing in eligible PsO patients, with no new or unexpected safety signals*4
*The recommended dose is Cosentyx 300 mg SC with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing.1
Based on clinical response, a maintenance dose of 300 mg every 2 weeks may provide additional benefit for patients with a body weight of 90 kg or higher.1
Please read full warnings and precautions (found in the SmPC) when prescribing Cosentyx.
Download a summary of the Cosentyx safety profile
Summary from clinical trials and post-marketing experience1
Corresponding frequency category for each adverse drug reaction is based on the following convention: very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000); and not known (cannot be estimated from the available data).
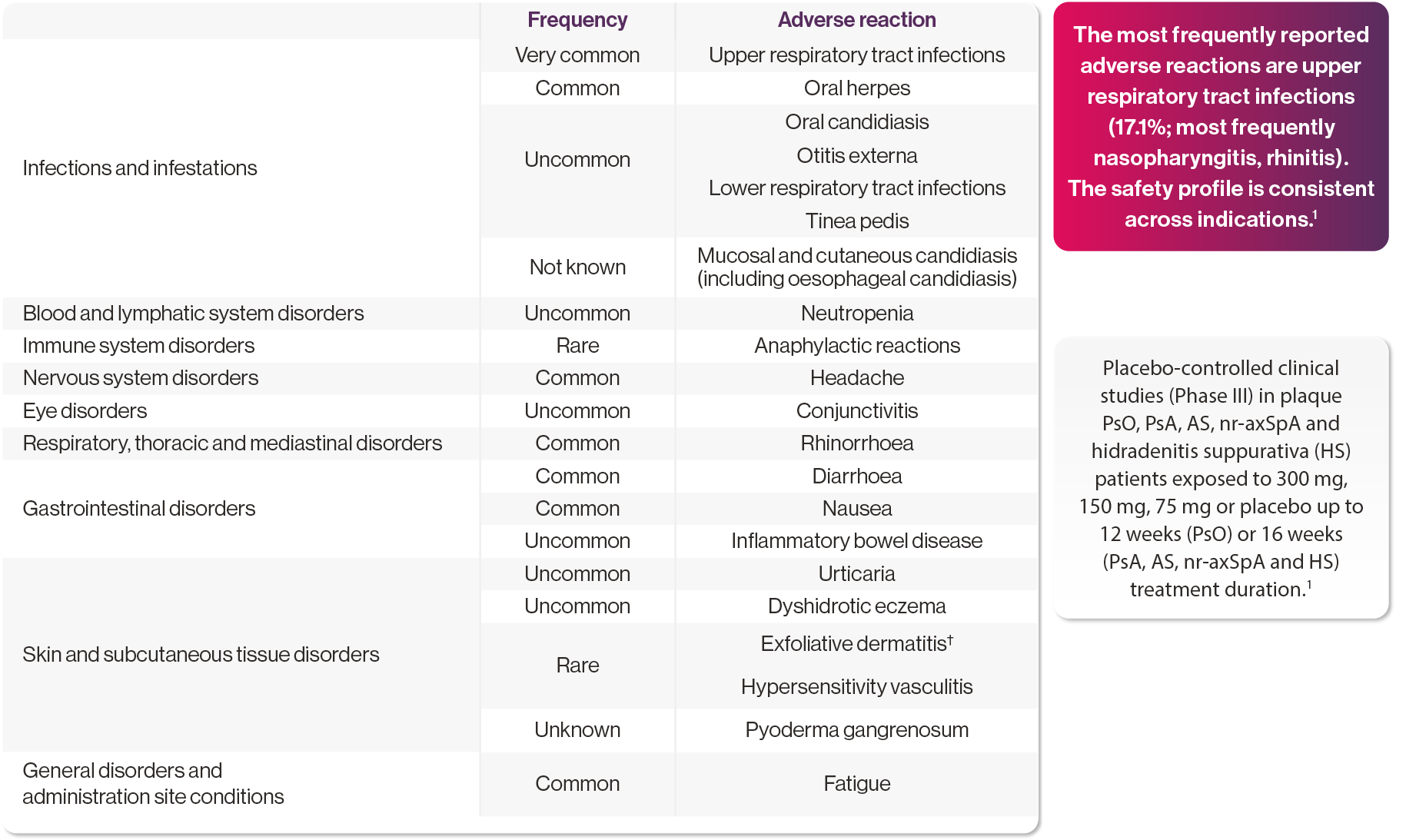
Adapted from Cosentyx SmPC. Please refer to the SmPC for full information on adverse events.1
†Cases were reported in patients with psoriasis diagnosis.1
Cosentyx has a 5-year consistent safety profile across 28 studies in PsO and PsA3
| PsO | PsA |
|---|---|---|
Most common AEs, EAIR per 100 PY | ||
Nasopharyngitis | 22.6 | 11.6 |
Headache | 7.3 | 3.8 |
Diarrhoea | 4.2 | 3.9 |
Upper respiratory tract infection | 5.3 | 8.8 |
AEs of special interest, EAIR per 100 PY | ||
Serious infections† | 1.4 | 1.8 |
Candida infections‡ | 2.9 | 1.5 |
Opportunistic infections§ | 0.19 | 0.18 |
IBD¶ | 0.01 | 0.03 |
Crohn’s disease¶ | 0.1 | 0.1 |
Ulcerative colitis¶ | 0.01 | 0.1 |
MACE‖ | 0.4 | 0.4 |
Uveitis¶ | 0.01 | 0.1 |
Malignancy** | 0.9 | 1.0 |
Table adapted from Gottlieb AB, et al. 2022.3
†Rates for system organ class.3
‡Rates for high-level terms.3
§Opportunistic infections were bronchopulmonary aspergillosis, cytomegalovirus gastroenteritis, gastrointestinal candidiasis, herpes zoster cutaneous disseminated, herpes zoster infection neurological, Mycobacterium avium complex infection, oesophageal candidiasis, Pneumocystis jirovecii pneumonia, toxoplasmosis, tuberculosis.3
¶Rates for preferred terms.3
‖Rates for Novartis MedDRA Query terms.3
**Rates for standardised MedDRA Query terms—“malignancies and unspecified tumour”.3
Cases of IBD have been reported with Cosentyx; therefore, it is not recommended in patients with IBD. Cosentyx is contraindicated in patients with clinically important, active infection, e.g., active tuberculosis. Please refer to the SmPC for full safety information.1
Gottlieb et al: An integrated safety analysis including data from 28 clinical studies was used to report the long-term safety profile of Cosentyx in PsO, AS and PsA. Clinical studies (18 PsO, 4 AS and 5 PsA) and post-marketing safety surveillance data alongside the PSUR were included (N=12,637). Safety assessments included AEs, SAEs and AESIs. Incidences of SAEs were low, with no identifiable patterns across indications. No new safety signals were identified.3
Adverse events were similar across Q2W and Q4W treatment groups4
TEAEs, n (%) | Cosentyx | Cosentyx | Cosentyx | Total |
All AEs | 127 (77.0) | 97 (72.4) | 24 (77.4) | 248 (75.2) |
AEs possibly related to Cosentyx | 34 (20.6) | 29 (21.6) | 5 (16.1) | 68 (20.6) |
All nonfatal SAEs | 14 (8.5) | 17 (12.7) | 4 (12.9) | 35 (10.6) |
Deaths | 0 (0.0) | 1 (0.7) | 0 (0.0) | 1 (0.3) |
Discontinued study treatment due to AEs | 4 (2.4) | 9 (6.7) | 2 (6.5) | 15 (4.5) |
Treatment-emergent SAEs by system organ class term‡ | ||||
Infections and infestations | 1 (0.6) | 6 (4.5) | 2 (6.5) | 9 (2.7) |
Injury, poisoning and procedural complications | 3 (1.8) | 3 (2.2) | 1 (3.2) | 7 (2.1) |
Gastrointestinal disorders | 0 (0.0) | 4 (3.0) | 1 (3.2) | 5 (1.5) |
Cardiac disorders | 1 (0.6) | 3 (2.2) | 1 (3.2) | 5 (1.5) |
Respiratory, thoracic and mediastinal disorders | 3 (1.8) | 1 (0.7) | 0 (0.0) | 4 (1.2) |
Musculoskeletal and connective tissue disorders | 3 (1.8) | 0 (0.0) | 0 (0.0) | 3 (0.9) |
General disorders and administration site conditions | 2 (1.2) | 1 (0.7) | 0 (0.0) | 3 (0.9) |
Most frequent AEs§ by preferred term | ||||
Nasopharyngitis | 32 (19.4) | 22 (16.4) | 5 (16.1) | 59 (17.9) |
Upper respiratory tract infection | 12 (7.3) | 9 (6.7) | 3 (9.7) | 24 (7.3) |
Headache | 11 (6.7) | 6 (4.5) | 1 (3.2) | 18 (5.5) |
Diarrhoea | 10 (6.1) | 6 (4.5) | 2 (6.5) | 18 (5.5) |
Arthralgia | 7 (4.2) | 6 (4.5) | 2 (6.5) | 15 (4.5) |
Oropharyngeal pain | 3 (1.8) | 7 (5.2) | 2 (6.5) | 12 (3.6) |
Cough | 7 (4.2) | 2 (1.5) | 2 (6.5) | 11 (3.3) |
Back pain | 3 (1.8) | 6 (4.5) | 2 (6.5) | 11 (3.3) |
AEs of special interest |
|
|
|
|
Infections and infestations (SOC) | 76 (46.1) | 63 (47.0) | 18 (58.1) | 157 (47.6) |
Hypersensitivity (SMQ narrow) | 14 (8.5) | 6 (4.5) | 0 (0.0) | 20 (6.1) |
Candida infections (HLT) | 3 (1.8) | 6 (4.5) | 1 (3.2) | 10 (3.0) |
Neutropenia (NMQ narrow) | 7 (4.2) | 5 (3.7) | 3 (9.7) | 15 (4.5) |
IBD (NMQ narrow) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
MACE (NMQ) | 0 (0.0) | 2 (1.5) | 0 (0.0) | 2 (0.6) |
Table adapted from Augustin M, et al. 2022.4
†One patient who did not receive any treatment after randomisation was excluded from the Q4W safety analyses.4
‡n ≥3 in the overall study population.4
§n ≥10 in the overall study population.4
Cases of IBD have been reported with Cosentyx; therefore, it is not recommended in patients with IBD. Cosentyx is contraindicated in patients with clinically important, active infection, e.g., active tuberculosis. Please refer to the SmPC for full safety information.1
Augustin et al: Multicentre, double-blind, parallel-group trial (N=331): adult patients with moderate to severe PsO weighing ≥90 kg were randomised to receive Cosentyx 300 mg Q2W or Q4W. The primary endpoint was PASI 90 response at Week 16. Secondary endpoints included the proportion of patients achieving an IGA mod 2011 score of 0 or 1 (indicating clear or almost clear skin) at Week 16, and clinical safety and tolerability measures (clinical laboratory parameters, vital signs, electrocardiograms and AEs) up to Week 52. At Week 16, Q2W dosing (n=165) led to significantly higher PASI 90 responses vs Q4W (n=166) with 73.2% vs 55.5%; p=0.0003, (OR: 2.3; 95% CI: 1.4–3.8).4
Data from SUNSHINE and SUNRISE shows a consistent safety profile in HS5
| SUNSHINE | SUNRISE | ||||
Outcome (Week 16) | Cosentyx | Cosentyx | Placebo | Cosentyx | Cosentyx | Placebo |
Patients with any AEs, n (%) | 122 (67) | 118 (66) | 120 (67) | 113 (63) | 114 (63) | 116 (63) |
Most common AEs by preferred term, n (%) | ||||||
Headache | 17 (19) | 20 (11) | 14 (8) | 21 (12) | 17 (9) | 15 (8) |
Nasopharyngitis | 20 (11) | 16 (9) | 13 (7) | 13 (7) | 9 (5) | 16 (9) |
Hidradenitis | 11 (6) | 5 (3) | 24 (13) | 10 (6) | 11 (6) | 14 (8) |
Most common serious AEs by preferred term (two or more events in any group) | ||||||
Hidradenitis | 1 (1) | 0 (0) | 2 (1) | 1 (1) | 0 (0) | 0 (0) |
Patients with serious or other significant events, n (%) | ||||||
Deaths | 0 | 0 | 0 | 0 | 0 | 0 |
Non-fatal SAEs | 3 (2) | 3 (2) | 6 (3) | 6 (3) | 6 (3) | 5 (3) |
Discontinued study | 5 (3) | 1 (1) | 1 (1) | 1 (1) | 4 (2) | 4 (2) |
AEs of special interest, n (%) | ||||||
Infections and infestations | 59 (33) | 51 (28) | 53 (29) | 52 (29) | 59 (33) | 62 (34) |
URTI | 33 (18) | 26 (14) | 22 (12) | 27 (15) | 21 (12) | 29 (16) |
Fungal infectious disorders | 12 (7) | 1 (1) | 7 (4) | 7 (4) | 13 (7) | 3 (2) |
Candida infections | 2 (1) | 1 (1) | 4 (2) | 5 (3) | 5 (3) | 1 (1) |
Hypersensitivity | 12 (7) | 9 (5) | 9 (5) | 7 (4) | 5 (3) | 7 (4) |
IBD† | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1 (1) | 0 (0) |
Malignant or unspecified tumours‡ | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 1 (1) | 1 (1) |
MACE | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Adapted from Kimball AB, et al. 2023.5
†No increased risk of TB has been seen with Cosentyx in clinical trials.1
‡One case of inflammatory bowel disease and one case of ulcerative colitis were reported.5
Cases of IBD have been reported with Cosentyx; therefore, it is not recommended in patients with IBD. Cosentyx is contraindicated in patients with clinically important, active infection, e.g., active tuberculosis. Please refer to the SmPC for full safety information.1
SUNSHINE and SUNRISE: Identical, multicentre, randomised, placebo-controlled, double-blind Phase III trials. They were conducted in 219 primary sites in 40 countries. Patients (SUNSHINE N=676; SUNRISE N=687) were randomised to receive 300 mg Cosentyx every 2 weeks (Cosentyx Q2W group), Cosentyx 300 mg every 4 weeks (Cosentyx Q4W group) or placebo (placebo group) in a double-dummy fashion as per treatment assignment. Efficacy assessments included HS clinical response, abscess and inflammatory nodule count, flares and skin pain. The primary objective of both trials was clinical efficacy at Week 16. Secondary endpoints included mean percentage change from baseline in abscess and inflammatory nodule count at Week 16, the proportion of patients with flares over 16 weeks and the proportion of patients at Week 16 with a 30% or more reduction and reduction of two units or more of skin pain on a continuous numeric rating scale (NRS30) (assessed in patients with a baseline numeric rating of three or more). For both groups, the primary endpoint was met in the Cosentyx Q2W group. In SUNRISE, the Q4W also met the primary endpoints. In SUNSHINE, the primary endpoint was not met in the Cosentyx Q4W group.5
Cosentyx safety considerations1
Cosentyx is intended for use under the guidance and supervision of a physician experienced in the diagnosis and treatment of conditions for which Cosentyx is indicated. Please refer to the Cosentyx SmPC for full product information before prescribing.1
Therapeutic indications1
Cosentyx is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adult patients (alone or in combination with methotrexate [MTX]) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active ankylosing spondylitis (AS) in adults who have responded inadequately to conventional therapy; active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation as indicated by elevated C-reactive protein and/or magnetic resonance imaging evidence in adults who have responded inadequately to non-steroidal anti-inflammatory drugs; active moderate to severe hidradenitis suppurativa (HS; acne inversa) in adults with an inadequate response to conventional systemic HS therapy; active enthesitis-related arthritis (ERA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active juvenile psoriatic arthritis (JPsA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.
AE, adverse event; AESI, adverse event of special interest; AS, ankylosing spondylitis; CI, confidence interval; CNS, central nervous system; EAIR, exposure-adjusted incidence rate; ERA, enthesitis-realted arthritis; HLT, high-level term; HS, hidradenitis suppurativa; IBD, inflammatory bowel disease; IGA, investigator global assessment; JPsA, juvenile psoriatic arthritis; MACE, major adverse cardiovascular event; MedDRA, Medical Dictionary for Regulatory Activities; MTX, methotrexate; NMQ, Novartis MedRA Query; NR, non-responder; nr-axSpA, non-radiographic axial spondyloarthritis; OR, odds ratio; PASI, psoriasis area and severity index; PsA, psoriatic arthritis; PsO, plaque psoriasis; PSUR, periodic safety update report; PY, patient-year; Q2W, every 2 weeks; Q4W, every 4 weeks; SAE, serious adverse event; SC, subcutaneous; SmPC, summary of product characteristics; SMQ, standardised MedRA Query; SOC, system organ class; TB, tuberculosis; TNF, tumor necrosis factor.
References
Cosentyx® (secukinumab) Summary of Product Characteristics.
Deodhar A, et al. Arthritis Res Ther 2019;21(1):111.
Gottlieb AB, et al. Acta Derm Venereol 2022;102:adv00698.
Augustin M, et al. Br J Dermatol 2022;186(6):942–954.
Kimball AB, et al. Lancet 2023;401(10378):747–761
UK | January 2025 | FA-11328326
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.











