Cosentyx® (secukinumab): Dosing
Cosentyx is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adult patients (alone or in combination with methotrexate [MTX]) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active ankylosing spondylitis (AS) in adults who have responded inadequately to conventional therapy; active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation as indicated by elevated C-reactive protein and/or magnetic resonance imaging evidence in adults who have responded inadequately to nonsteroidal anti-inflammatory drugs; active enthesitis-related arthritis (ERA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active juvenile psoriatic arthritis (JPsA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.1
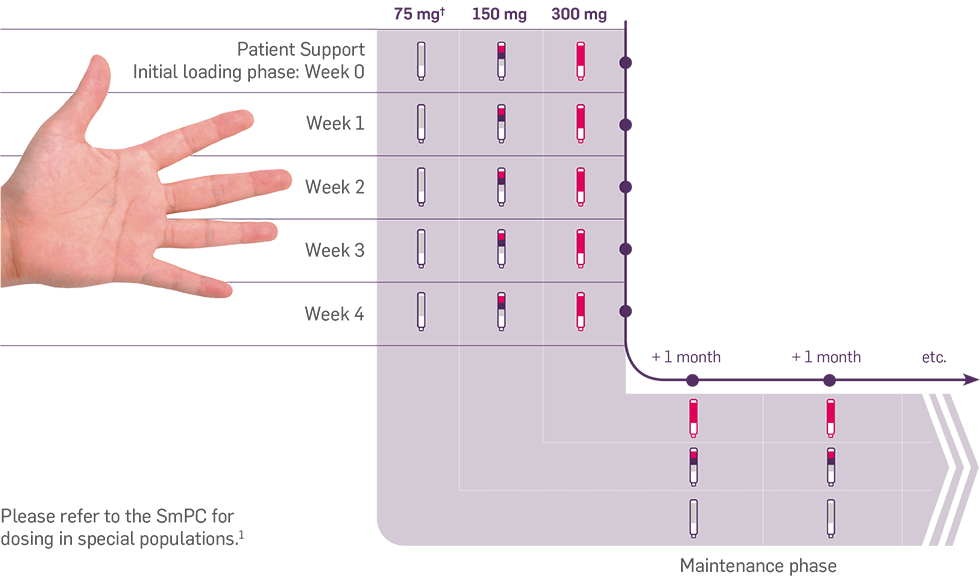
Convenience of a monthly injection with Cosentyx after initial loading doses*1
The ‘hand rule’ of Cosentyx dosing
Cosentyx is administered by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing.1 After proper training in subcutaneous injection technique, patients may self-inject Cosentyx or be injected by a caregiver if a physician determines that this is appropriate. The physician should ensure appropriate follow-up of patients. Patients or caregivers should be instructed to inject the full amount of Cosentyx according to the instructions provided in the package leaflet.1
Depending on the patient dosing regimen, the dosing schedule may look something like this:
Find out more about Cosentyx dosing
Consideration should be given to discontinuing treatment in patients who have shown no response by 16 weeks of treatment. Some patients with an initial partial response may subsequently improve with continued treatment beyond 16 weeks.1
Convenience of flexible dosing tailored to your eligible patients’ needs*1
Following the appropriate loading regimen:
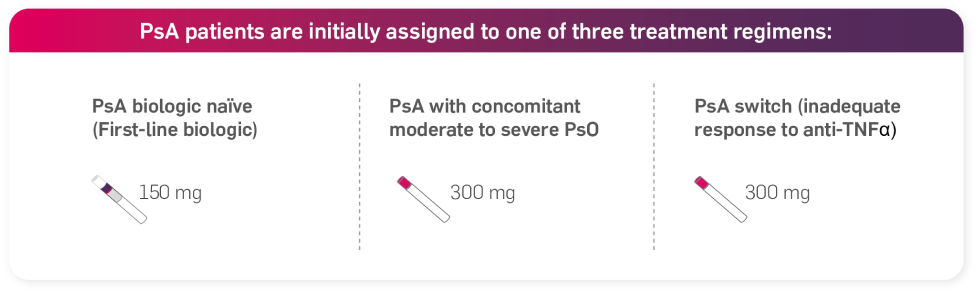
Psoriatic arthritis (PsA) alone or in combination with MTX
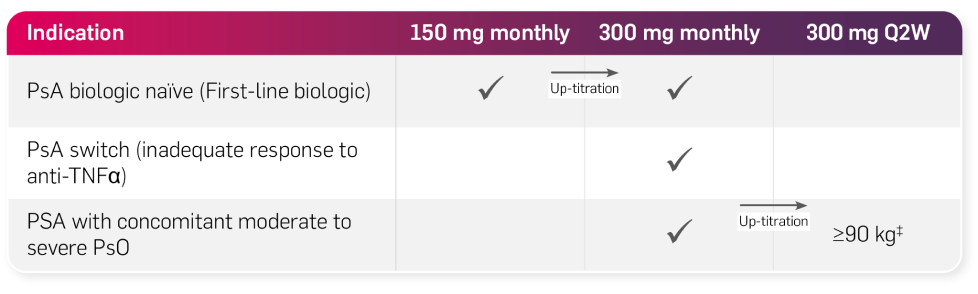
Depending on a patient’s diagnosis and clinical response, up-titration of Cosentyx may be prescribed at any point during the maintenance phase.1 After proper training in subcutaneous injection technique, eligible patients may self-inject Cosentyx or be injected by a caregiver if a physician determines that this is appropriate. The physician should ensure appropriate follow-up of patients. Patients or caregivers should be instructed to inject the full amount of Cosentyx according to the instructions provided in the package leaflet.1
Additional information for full posology in PsA
For patients with concomitant moderate to severe plaque psoriasis, the recommended dose is 300 mg of Cosentyx by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing. Based on clinical response, a maintenance dose of 300 mg every 2 weeks may provide additional benefit for patients with a body weight of 90 kg or higher.1
For patients who are anti-TNFα inadequate responders (IR), the recommended dose is 300 mg by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing.1
Each 300 mg dose is given as one subcutaneous injection of 300 mg or as two subcutaneous injections of 150 mg.1
For other patients, the recommended dose is 150 mg by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing. Based on clinical response, the dose can be increased to 300 mg.1
Special populations
Elderly patients (aged 65 years and over): No dose adjustment is required.1
Renal impairment/hepatic impairment: Cosentyx has not been studied in these patient populations. No dose recommendations can be made.1
Paediatric population: The safety and efficacy of Cosentyx in children with plaque psoriasis and in the JIA categories of ERA and JPsA below the age of 6 years have not been established.
The safety and efficacy of Cosentyx in children below the age of 18 years in other indications have not yet been established. No data are available.1
Flexible dosing based on your patients’ needs with Cosentyx*1
AxSpA patients are initially assigned:

Axial spondyloarthritis (AxSpA)
Following the appropriate loading regimen:

Depending on a patient’s diagnosis and clinical response, up-titration of Cosentyx may be prescribed at any point during the maintenance phase.1
After proper training in subcutaneous injection technique, patients may self-inject Cosentyx or be injected by a caregiver if a physician determines that this is appropriate. The physician should ensure appropriate follow-up of patients. Patients or caregivers should be instructed to inject the full amount of Cosentyx according to the instructions provided in the package leaflet.1
Additional information for full posology in axSpA
Ankylosing spondylitis (AS, radiographic axial spondyloarthritis): The recommended dose is 150 mg by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing. Based on clinical response, the dose can be increased to 300 mg.1
Each 300 mg dose is given as one subcutaneous injection of 300 mg or as two subcutaneous injections of 150 mg.1
Non-radiographic axial spondyloarthritis (nr-axSpA): The recommended dose is 150 mg by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing.1
Special populations
Elderly patients (aged 65 years and over): No dose adjustment is required.1
Renal impairment/hepatic impairment: Cosentyx has not been studied in these patient populations. No dose recommendations can be made.1
Paediatric population: The safety and efficacy of Cosentyx in children with plaque psoriasis and in the JIA categories of ERA and JPsA below the age of 6 years have not been established.
The safety and efficacy of Cosentyx in children below the age of 18 years in other indications have not yet been established. No data are available.1
Dosing in JIA patients*
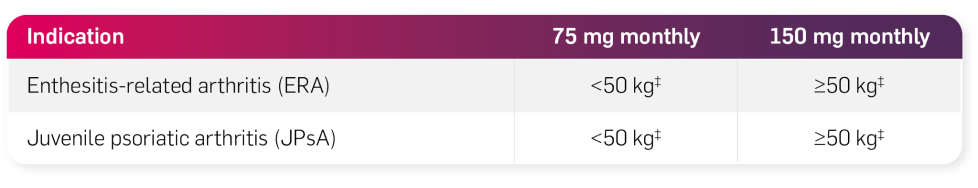
Juvenile idiopathic arthritis (JIA) alone or in combination with MTX
Following the appropriate loading regimen:
The 150 mg and 300 mg solution for injection in pre-filled syringe and in pre-filled pen are not indicated for administration to paediatric patients with a weight <50 kg. Cosentyx may be available in other strengths and/or presentations depending on the individual treatment needs.1
Additional information for full posology in JIA
The recommended dose is based on body weight (as per the table below) and administered by subcutaneous injection at Weeks 0, 1, 2, 3, and 4, followed by monthly maintenance dosing.1

The 150 mg and 300 mg solution for injection in pre-filled syringe and in pre-filled pen are not indicated for administration to paediatric patients with a weight <50 kg. Cosentyx may be available in other strengths and/or presentations depending on the individual treatment needs.1
Special populations
Renal impairment/hepatic impairment: Cosentyx has not been studied in these patient populations. No dose recommendations can be made.1
Paediatric population: The safety and efficacy of Cosentyx in children with JIA below the age of 6 years have not been established.1
Flexibility to dose every 2 weeks for patients with PsA and concomitant PsO*1
For adult patients with PsA and concomitant moderate to severe PsO, the recommended dose is 300 mg of Cosentyx by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing.1
Based on weight and clinical response, a maintenance dose of 300 mg every 2 weeks may provide additional benefit for adult patients with a body weight ≥90 kg. Each 300 mg dose is given as one subcutaneous injection of 300 mg or as two subcutaneous injections of 150 mg.1
Body weight ≥90 kg

Body weight <90 kg

Additional information for full posology in PsA with PsO
For patients with concomitant moderate to severe plaque psoriasis, the recommended dose is 300 mg of Cosentyx by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing. Based on clinical response, a maintenance dose of 300 mg every 2 weeks may provide additional benefit for patients with a body weight of 90 kg or higher.1
For patients who are anti-TNFα inadequate responders (IR), the recommended dose is 300 mg by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing.1
Each 300 mg dose is given as one subcutaneous injection of 300 mg or as two subcutaneous injections of 150 mg.1
For other patients, the recommended dose is 150 mg by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing. Based on clinical response, the dose can be increased to 300 mg.1
Special populations
Elderly patients (aged 65 years and over): No dose adjustment is required.1
Renal impairment/hepatic impairment: Cosentyx has not been studied in these patient populations. No dose recommendations can be made.1
Paediatric population: The safety and efficacy of Cosentyx in children with plaque psoriasis and in the JIA categories of ERA and JPsA below the age of 6 years have not been established.
The safety and efficacy of Cosentyx in children below the age of 18 years in other indications have not yet been established. No data are available.1
Cosentyx is intended for use under the guidance and supervision of a physician experienced in the diagnosis and treatment of conditions for which Cosentyx is indicated. Please refer to the Cosentyx SmPC for full product information and administration, including dosing in special populations, before prescribing.1 Cosentyx has not been studied in patients with renal impairment or hepatic impairment. No dose recommendations can be made.
Therapeutic Indications1
Cosentyx is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adult patients (alone or in combination with methotrexate [MTX]) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active ankylosing spondylitis (AS) in adults who have responded inadequately to conventional therapy; active nonradiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation as indicated by elevated C-reactive protein and/or magnetic resonance imaging evidence in adults who have responded inadequately to non-steroidal anti-inflammatory drugs; active moderate to severe hidradenitis suppurativa (HS; acne inversa) in adults with an inadequate response to conventional systemic HS therapy; active enthesitis-related arthritis (ERA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active juvenile psoriatic arthritis (JPsA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.1
*Individual dosing may vary – please consult the SmPC for further information.1
†Pre-filled syringe for paediatric patients.1
‡Bodyweight at time of dosing.1
AS, ankylosing spondylitis; axSpA, axial spondyloarthritis; CRP, C-reactive protein; DMARD, disease modifying anti-rheumatic drug; ERA, enthesitis-related arthritis; HS, hidradenitis suppurativa; JIA, juvenile idiopathic arthritis; JPsA, juvenile psoriatic arthritis; MRI, magnetic resonance imaging; MTX, methotrexate; nr-axSpA, non-radiographic axial spondyloarthritis; PsA, psoriatic arthritis; PsO, plaque psoriasis; Q2W, every 2 weeks; SmPC, summary of product characteristics; TNFα, tumour necrosis factor alpha.
Reference
Cosentyx® (secukinumab) Summary of Product Characteristics.
UK | January 2025 | FA-11340736
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.