

Cosentyx® (secukinumab) hidradenitis suppurativa dosing
Cosentyx is indicated for the treatment of active moderate to severe hidradenitis suppurative (HS) in adults with an inadequate response to conventional systemic HS therapy.1
Cosentyx is intended for use under the guidance and supervision of a physician experienced in the diagnosis and treatment of conditions for which Cosentyx is indicated.1
Please refer to the Cosentyx Summary of Product Characteristics for full prescribing details, safety information, administration and dosing including in special populations.1
Convenience of a monthly injection with Cosentyx after initial loading doses1
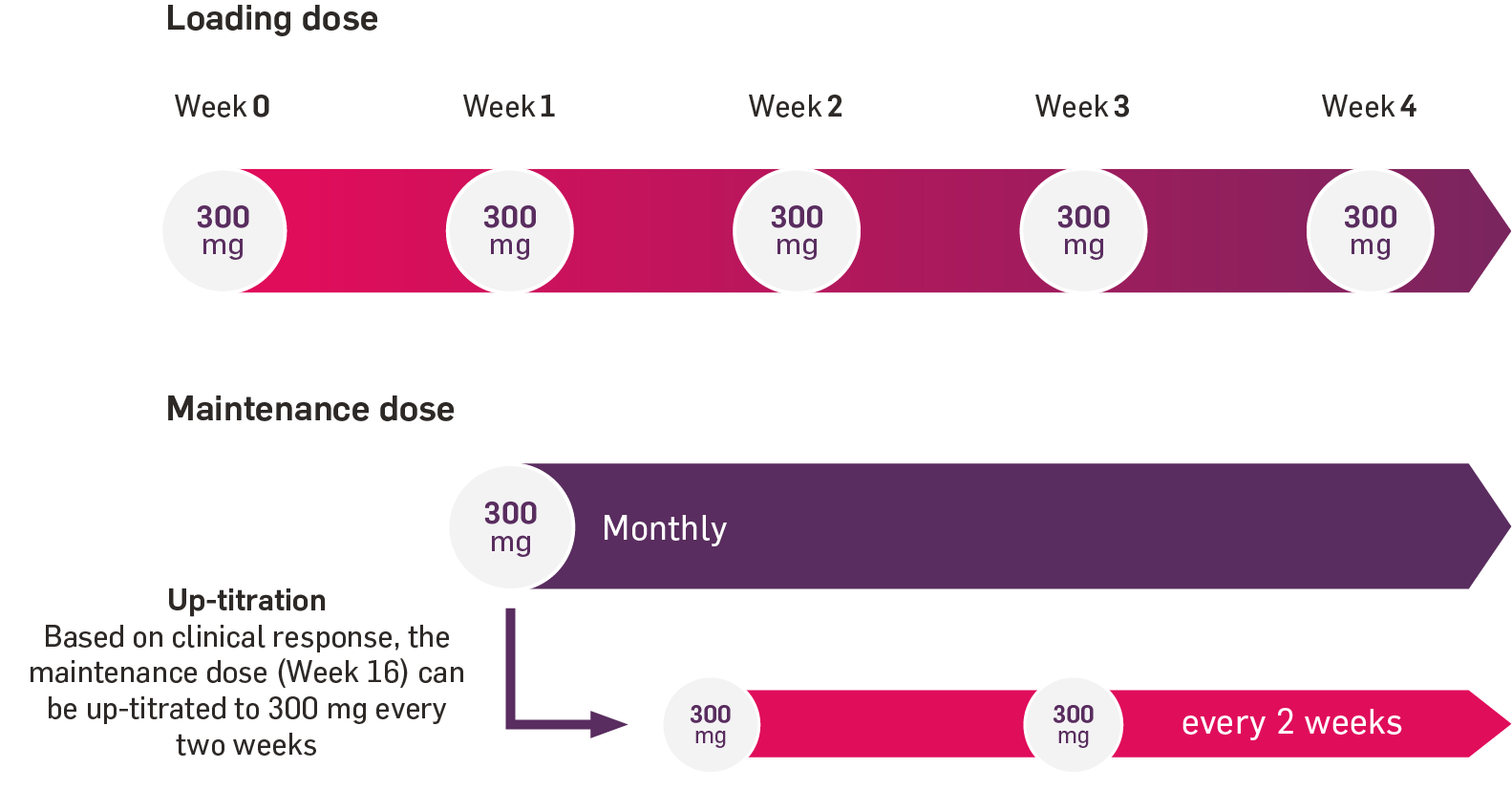
The recommended dose of Cosentyx in adults with HS is 300 mg delivered at Weeks 0, 1, 2, 3 and 4, followed by a monthly maintenance dose.1
Based on clinical response, the maintenance dose can be up-titrated to 300 mg every two weeks. Each 300 mg dose is given as one subcutaneous injection of 300 mg or as two subcutaneous injections of 150 mg.1
Individual dosing may vary – please consult the SmPC for further information.1
Explore the QoL data for both Q2W and Q4W dosing regimens with Cosentyx in patients with HS
Cosentyx pre-filled pens for HS
Cosentyx pre-filled pens are available in two doses: 150 mg and 300 mg.1,2 Click on the images below to watch the administration videos for the UnoReady® and SensoReady® pens.
Cosentyx is to be administered by subcutaneous injection. If possible, areas of the skin that show psoriasis should be avoided as injection sites. The pen must not be shaken. After proper training in subcutaneous injection technique, patients may self-inject Cosentyx or be injected by a caregiver if a physician determines that this is appropriate. However, the physician should ensure appropriate follow-up of patients. Patients or caregivers should be instructed to inject the full amount of Cosentyx according to the instructions provided in the package leaflet. Comprehensive instructions for administration are given in the package leaflet.1
Cosentyx dosing precautions and considerations
The efficacy of Cosentyx in HS has been established through clinical studies. Available evidence suggests that a clinical response is normally achieved within the first 16 weeks of treatment; however, if your patients don’t demonstrate a response to the prescribed Cosentyx dose within this timeframe, consideration should be given to discontinuing the treatment. Some patients with an initial partial response may subsequently improve with continued treatment beyond 16 weeks.1
This is not an exhaustive list of warnings and precautions. Please refer to the Cosentyx SmPC for full information.1
The most frequently reported adverse reactions are upper respiratory tract infections (17.1%) (most frequently nasopharyngitis, rhinitis).1
Therapeutic indications1
Cosentyx is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adult patients (alone or in combination with methotrexate [MTX]) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active ankylosing spondylitis (AS) in adults who have responded inadequately to conventional therapy; active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation as indicated by elevated C-reactive protein and/or magnetic resonance imaging evidence in adults who have responded inadequately to non-steroidal anti-inflammatory drugs; active moderate to severe hidradenitis suppurativa (HS; acne inversa) in adults with an inadequate response to conventional systemic HS therapy; active enthesitis-related arthritis (ERA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active juvenile psoriatic arthritis (JPsA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.1
AS, ankylosing spondylitis; ERA, enthesitis-related arthritis; HS, hidradenitis suppurativa; JPsA, juvenile psoriatic arthritis; MTX, methotrexate; nr-axSpA, non-radiographic axial spondyloarthritis; PsA, psoriatic arthritis; PsO, plaque psoriasis; Q2W, every 2 weeks; Q4W, every 4 weeks; QoL, quality of life; SmPC, summary of product characteristics.
References
Cosentyx® (secukinumab) Summary of Product Characteristics.
Cosentyx® (secukinumab) Patient Information Leaflet.
UK | January 2025 | FA-11252576
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.







