

SCEMBLIX®▼ (asciminib) is indicated for the treatment of adult patients with Philadelphia chromosome-positive chronic myeloid leukaemia (Ph + CML) in chronic phase (CP), previously treated with two or more tyrosine kinase inhibitors, and without a known T315I mutation.1
The unmet need in chronic myeloid leukaemia
Tyrosine kinase inhibitors (TKIs) have improved CML prognosis, yet survival remains poor in patients on ≥3rd line therapy.2,3
Many patients on TKIs face treatment failure or discontinuation due to intolerance, with up to half of patients discontinuing 1st line imatinib within 5 years.3
During 2nd-line treatment:3

of patients fail to achieve major molecular response (MMR) (n=1279)*3
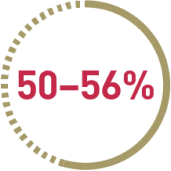
of patients fail to achieve complete cytogenetic response (CCyR) within 2 years (n=958)*3
*Results for MMR and CCyR rates are taken from multiple studies including different 2nd line medications. This is a meta-analysis.3
In CML patients, a low 8-year overall survival was associated with being on ≥3rd line treatment.†2
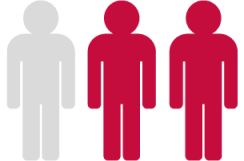
In approximately 2/3 of those who had at least 1 TKI switch it was due to resistance†4 (n=73/113)
8-year overall survival, N=902
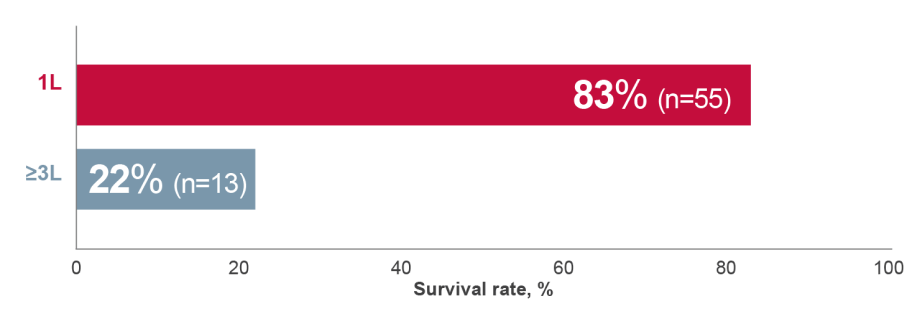
Adapted from Bosi GR, et al. 2019.2
A 2nd generation TKI may have limited benefit in a ≥3L setting, after the failure of another 2nd generation TKI and 1st generation TKI prior.3 Patients in ≥3L treatment may need a different mechanism of action to optimise outcomes.2,7–9
“You can end up running out of options for patients who experience side effects. Then you need to consider transplant after 4th line or chemotherapy options”
Adapted from haematologist quote11
“In a year I had gone from taking one drug and living a normal life to having zero options”
Adapted from 5L CML patient quote12
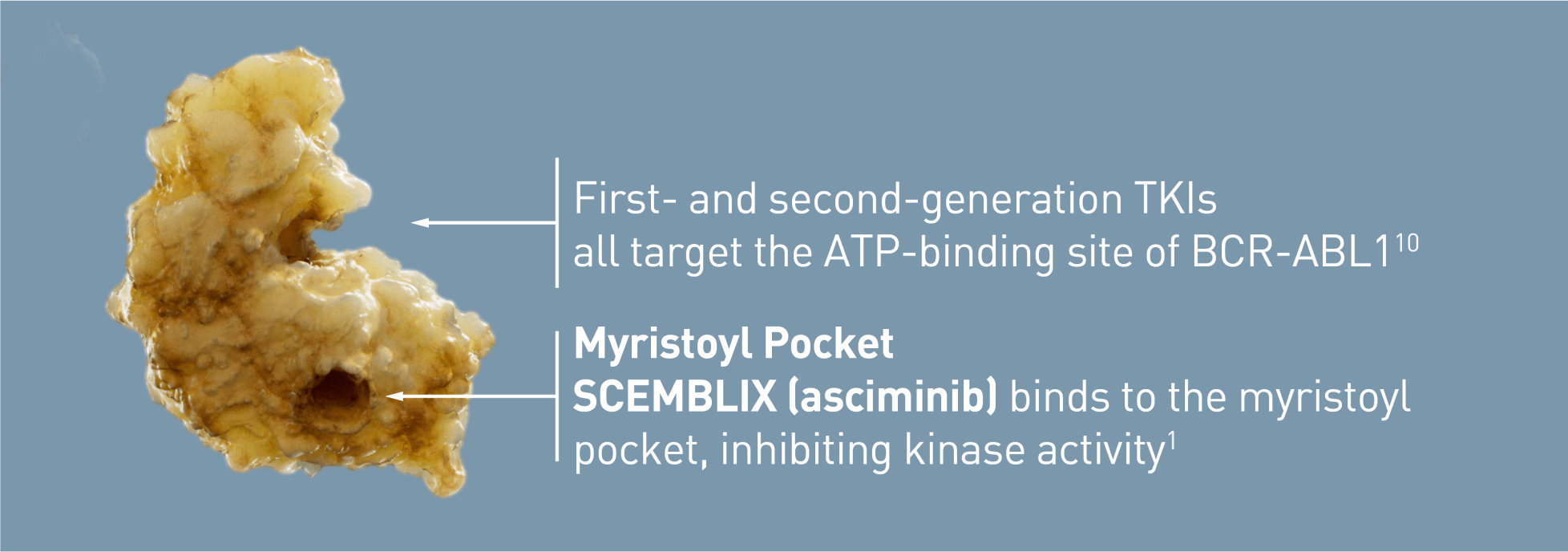
SCEMBLIX is the first and only STAMP inhibitor, specifically targeting the ABL1 myristoyl pocket10,13,14
The ASCEMBL trial did not restrict to Ph+ patients with CP-CML. SCEMBLIX is indicated in adults with Ph+ CP-CML previously treated with two or more tyrosine kinase inhibitors and without a known T315I mutation.1,13
†Data from a retrospective, non-interventional study conducted at 21 UK NHS secondary and tertiary care centres on 257 patients with CML.4
1L, first line; 3L, third line; ATP, adenosine triphosphate; BCR-ABL, breakpoint cluster region and Abelson murine leukaemia viral oncogene homologue; CCyR, complete cytogenetic response; CI, confidence interval; CML, chronic myeloid leukaemia; CP, chronic phase; MCyR, major cytogenetic response; MMR, major molecular response; MOA, mechanism of action; NHS, National Health Service; Ph+, Philadelphia chromosome positive; STAMP, specifically targeting the ABL1 myristoyl pocket; TKI, tyrosine kinase inhibitor.
For further information, please refer to the Summary of Product Characteristics.
References
SCEMBLIX (asciminib) Summary of Product Characteristics.
Bosi GR, et al. Hematol Transfus Cell Ther 2019;41(3):222–228.
Cortes JE and Lang F. J Hematol Oncol 2021;14(1):44.
Milojkovic D, et al. Br J Haematol 2021;192:62–74.
Smith G, et al. Br J Haematol 2020;191:171–193.
Hochhaus A, et al. Leukaemia 2020;34:1495–1502.
Soverini S, et al. Blood 2009;114(10):2168–2171.
Garg RJ, et al. Blood 2009;114(20):4361–4368.
Ibrahim AR, et al. Blood 2010;116(25):5497–5500.
Schoepfer J, et al. J Med Chem 2018;61(18):8120–8135.
Novartis data on file; Asc001.
Novartis data on file; Hae003.
Réa D, et al. Blood 2021;138(21):2031–2041.
Redaelli S, et al. J Clin Oncol 2009;27(3):469–471.
UK | November 2024 | FA-11311664
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.
