

Cosentyx® (secukinumab): Real-world experience with flexible dosing in eligible patients
Cosentyx is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adult patients (alone or in combination with methotrexate [MTX]) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active ankylosing spondylitis (AS) in adults who have responded inadequately to conventional therapy; active nonradiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation as indicated by elevated C-reactive protein and/or magnetic resonance imaging evidence in adults who have responded inadequately to non-steroidal anti-inflammatory drugs; active moderate to severe hidradenitis suppurativa (HS; acne inversa) in adults with an inadequate response to conventional systemic HS therapy; active enthesitis-related arthritis (ERA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active juvenile psoriatic arthritis (JPsA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.1
For full indications, please refer to the Cosentyx Summary of Product Characteristics (SmPC).
Cosentyx has not been studied in patients with renal/hepatic impairment. No dose recommendations can be made.1
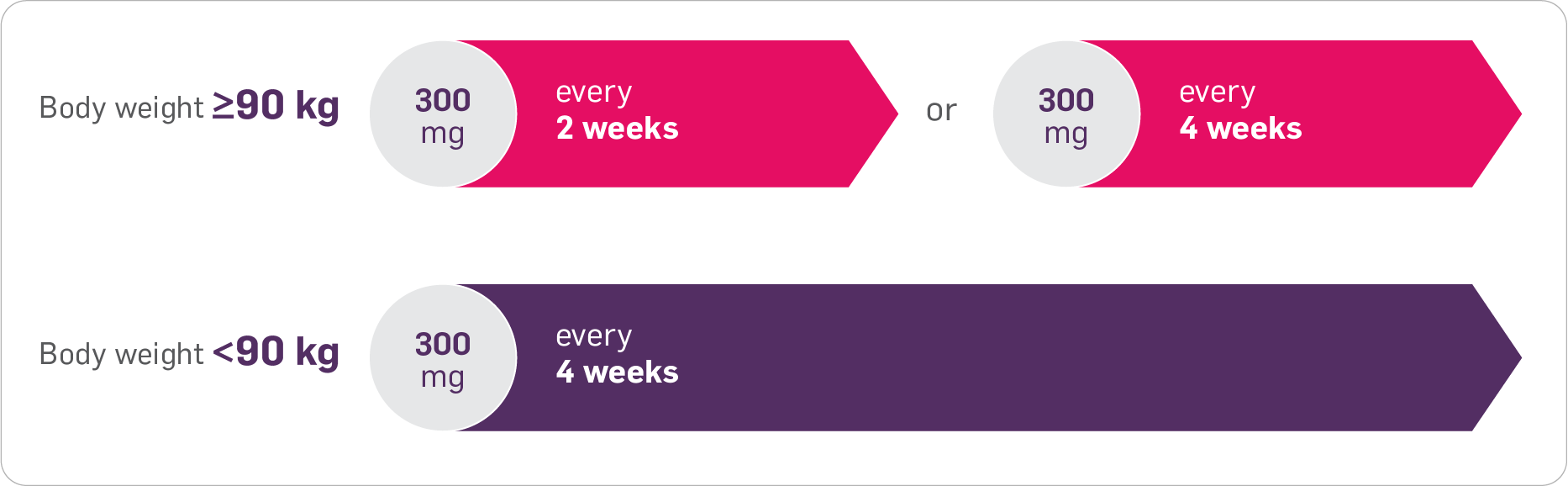
Cosentyx offers flexible dosing in eligible patients with PsA and concomitant PsO1
Eligible patients are those weighing ≥90 kg.
For adults with PsA and concomitant moderate to severe PsO, the recommended dose is 300 mg of Cosentyx by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing.1
Based on weight and clinical response, a maintenance dose of 300 mg every 2 weeks may provide additional benefit for adult patients with a body weight ≥90 kg. Each 300 mg dose is given as one subcutaneous injection of 300 mg or as two subcutaneous injections of 150 mg.1
Hear first-hand experiences of using Cosentyx flexible dosing in eligible patients in the real world
Eligible patients are those weighing ≥90 kg.
Watch the case studies
Explore two case studies showing real-world patients who have been prescribed Cosentyx, highlighting the clinical results of individualised treatment approaches.
Understand the different dosing options with Cosentyx
Cosentyx 300 mg Q2W dosing may help your eligible patients achieve fast and lasting skin clearance2
Fast = efficacy at 16 weeks and lasting = efficacy at 52 weeks.2
You can tailor the dosing of Cosentyx based on your PsO patients’ clinical response. In the A2324 Q2W clinical trial, FASTER and LONG-LASTING skin clearance was seen in patients (≥90 kg) treated with Cosentyx 300 mg Q2W vs 300 mg Q4W.2
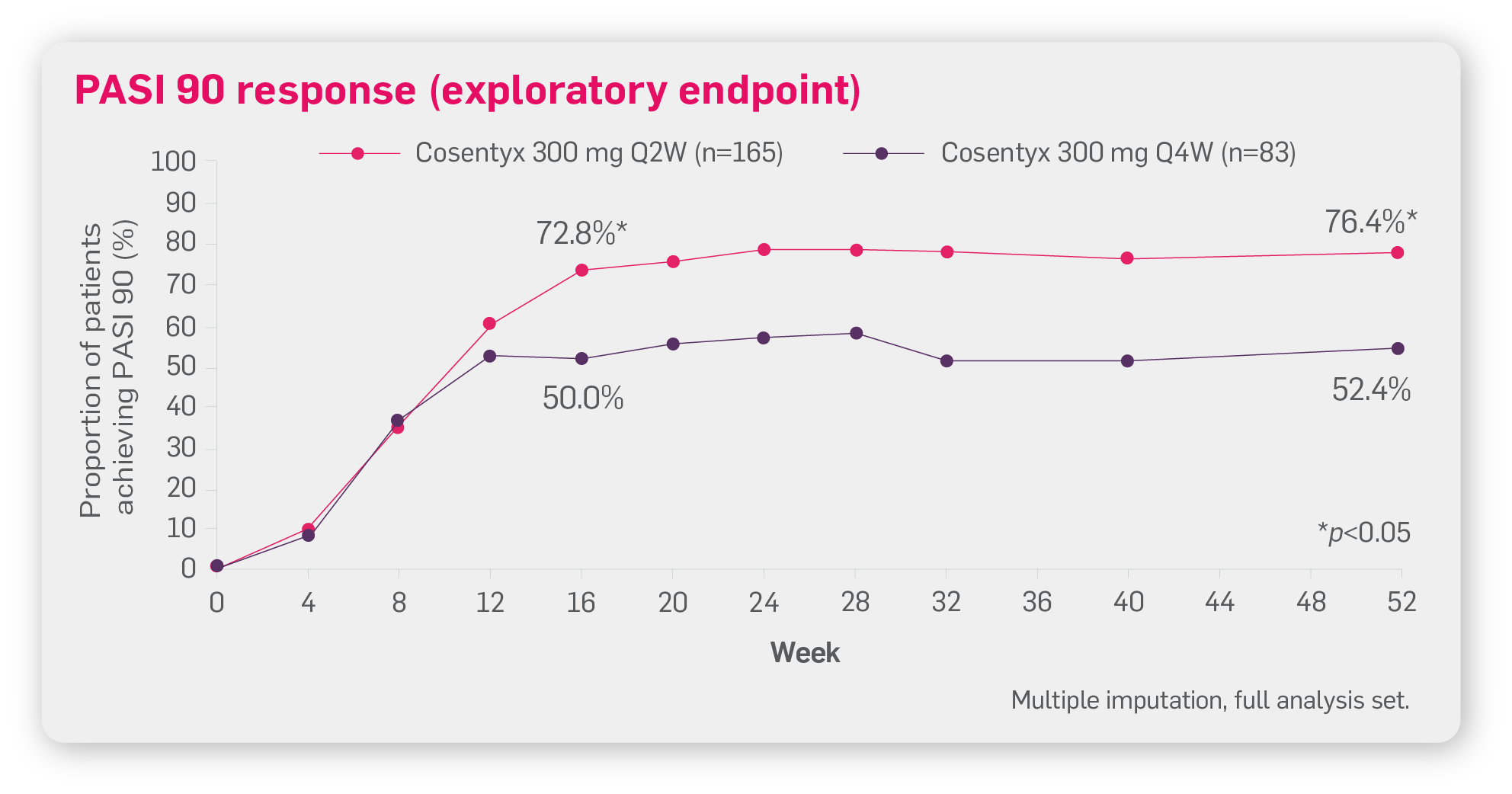
Adapted from Augustin M, et al. 2022.2
A2324 Q2W was a multicentre, double-blind, parallel-group trial on patients with moderate to severe PsO weighing ≥90 kg (N=331) treated with Cosentyx 300 mg Q2W or Q4W.*2
Primary endpoint of PASI 90 response at Week 16 for Cosentyx 300 mg Q2W vs Q4W was met.2
Please note the p value of p=0.0003 is related to the primary endpoint. For the exploratory endpoint at Week 52, the p value is <0.05.
Safety findings are comparable across Cosentyx 300 mg Q2W and Q4W regimes in patients weighing ≥90 kg2
| Treatment-emergent AEs, n (%) | Cosentyx | Cosentyx | Cosentyx 300 mg Q4W |
|---|---|---|---|
| All AEs | 127 (77.0) | 97 (72.4) | 24 (77.4) |
| AEs possibly related to Cosentyx | 34 (20.6) | 29 (21.6) | 5 (16.1) |
| All non-fatal SAEs | 14 (8.5) | 17 (12.7) | 4 (12.9) |
| Deaths‡ | 0 (0.0) | 1 (0.7) | 0 (0.0) |
| Discontinued study treatment due to AEs | 4 (2.4) | 9 (6.7) | 2 (6.5) |
| Treatment-emergent SAEs by SOC term§ | |||
| Infections and infestations | 1 (0.6) | 6 (4.5) | 2 (6.5) |
| Injury, poisoning and procedural complications | 3 (1.8) | 3 (2.2) | 1 (3.2) |
| Gastrointestinal disorders | 0 (0.0) | 4 (3.0) | 1 (3.2) |
| Cardiac disorders | 1 (0.6) | 3 (2.2) | 1 (3.2) |
| Respiratory, thoracic and mediastinal disorders | 3 (1.8) | 1 (0.7) | 0 (0.0) |
| Musculoskeletal and connective tissue disorders | 3 (1.8) | 0 (0.0) | 0 (0.0) |
| General disorders and administration site conditions | 2 (1.2) | 1 (0.7) | 0 (0.0) |
| Most frequent AEs by preferred termǁ | |||
| Nasopharyngitis | 32 (19.4) | 22 (16.4) | 5 (16.1) |
| Upper respiratory tract infection | 12 (7.3) | 9 (6.7) | 3 (9.7) |
| Headache | 11 (6.7) | 6 (4.5) | 1 (3.2) |
| Diarrhoea | 10 (6.1) | 6 (4.5) | 2 (6.5) |
| Arthralgia | 7 (4.2) | 6 (4.5) | 2 (6.5) |
| Oropharyngeal pain | 3 (1.8) | 7 (5.2) | 2 (6.5) |
| Cough | 7 (4.2) | 2 (1.5) | 2 (6.5) |
| Back pain | 3 (1.8) | 6 (4.5) | 2 (6.5) |
| AEs of special interest | |||
| Infections and infestations (SOC) | 76 (46.1) | 63 (47.0) | 18 (58.1) |
| Hypersensitivity (SMQ narrow) | 14 (8.5) | 6 (4.5) | 0 (0.0) |
| Candida infections (HLT) | 3 (1.8) | 6 (4.5) | 1 (3.2) |
| Neutropenia (NMQ narrow) | 7 (4.2) | 5 (3.7) | 3 (9.7) |
| IBD (NMQ narrow) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| MACE (NMQ) | 0 (0.0) | 2 (1.5) | 0 (0.0) |
Table adapted from Augustin M, et al. 2022.2
The most frequently reported adverse reactions are upper respiratory tract infections (17.1%) most frequently nasopharyngitis, rhinitis.1
Please refer to the SmPC for detailed safety data and full prescribing and administration information, including dosing in special populations and warnings/precautions.1
Cosentyx is intended for use under the guidance and supervision of a physician experienced in the diagnosis and treatment of conditions for which Cosentyx is indicated. Please refer to the Cosentyx SmPC for full product information before prescribing.1
Therapeutic Indications1
Cosentyx is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adult patients (alone or in combination with methotrexate [MTX]) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active ankylosing spondylitis (AS) in adults who have responded inadequately to conventional therapy; active nonradiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation as indicated by elevated C-reactive protein and/or magnetic resonance imaging evidence in adults who have responded inadequately to non-steroidal anti-inflammatory drugs; active moderate to severe hidradenitis suppurativa (HS; acne inversa) in adults with an inadequate response to conventional systemic HS therapy; active enthesitis-related arthritis (ERA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active juvenile psoriatic arthritis (JPsA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.1
*A2324 Q2W: A multicentre, double-blind, parallel group trial of patients weighing ≥90 kg (N=331) in patients with moderate to severe PsO. Patients received either Cosentyx 300 mg Q2W or Q4W. The primary endpoint was PASI 90 response at Week 16. At Week 16, Q2W led to significantly higher PASI 90 responses vs Q4W (n=166; 73.2% vs 55.5%; p=0.0003). Secondary endpoints were the proportion of patients achieving an IGA mod 2011 score of 0 or 1 (indicating clear or almost clear skin) at Week 16, and clinical safety and tolerability measures (clinical laboratory parameters, vital signs, electrocardiograms and adverse events) up to Week 52.2
†One patient who did not receive any study treatment after randomisation was excluded from the Q4W safety analyses.2
‡One death was reported in the Q4W group (patient was aged 83 years with ongoing medical conditions of hyperlipidemia, hypertension and aortic stenosis, and died during Treatment Period 2 of cardiac arrest/acute MI).2
§Number of patients ≥3 in the overall study population.2
‖Number of patients ≥10 in the overall study population.2
AE, adverse event; AS, ankylosing spondylitis; ERA, enthesitis-related arthritis; HLT, high-level term; HS, hidradenitis suppurativa; IBD, inflammatory bowel disease; JPsA, juvenile psoriatic arthritis; MACE, major adverse cardiovascular event; MI, myocardial infarction; MTX, methotrexate; NMQ, Novartis MedDRA Query; nr-axSpA, non-radiographic axial spondyloarthritis; PsA, psoriatic arthritis; PsO, plaque psoriasis; Q2W, every 2 weeks; Q4W, every 4 weeks; SAE, serious adverse event; SmPC, summary of product characteristics; SMQ, Standardised MedDRA Query; SOC, system organ class.
References
Cosentyx® (secukinumab) Summary of Product Characteristics.
Augustin M, et al. Br J Dermatol 2022;186(6):942–954.
UK | February 2025 | FA-11330277
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.



