JAKAVI® (ruxolitinib) in polycythaemia vera
JAKAVI® is indicated for the treatment of disease-related splenomegaly or symptoms in adult patients with primary myelofibrosis (also known as chronic idiopathic myelofibrosis), post polycythaemia vera myelofibrosis or post essential thrombocythaemia myelofibrosis. JAKAVI® is also indicated for adult patients with polycythaemia vera (PV) who are resistant to or intolerant of hydroxyurea.1
For your patients with polycythaemia vera who are resistant to or intolerant of hydroxyurea1
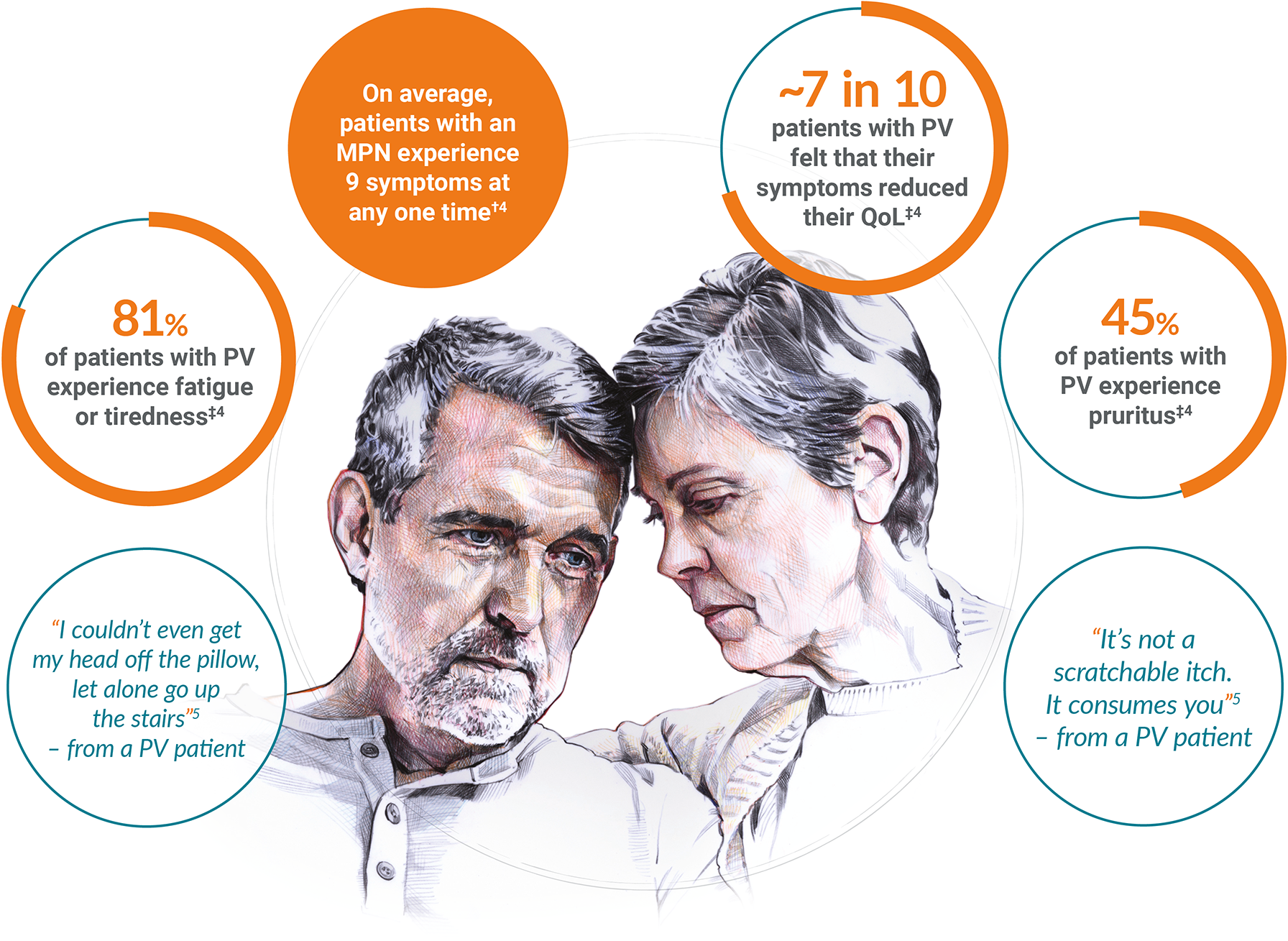
PV is a life-threatening condition associated with increased mortality and reduced quality of life, caused by a high symptom burden detrimentally impacting patients.2,3
~1 in 4 patients with a myeloproliferative neoplasm report that their condition interferes with or limits their daily activities.3 In the global MPN Landmark Survey (N=699), symptom reduction was reported as the primary goal of treatment by 61% of patients with PV.*3
The British Society for Haematology guidelines recommend a variety of first-, second- and third-line treatments for patients with PV.6 Hydroxyurea (also known as hydroxycarbamide) is commonly used as a first-line cytoreductive treatment in high-risk PV patients.7
Despite this, up to 20.7% of patients with PV may become intolerant or resistant to hydroxyurea treatment, which may lead to increased mortality.§8–10 Patients with PV who are HU resistant or intolerant are also at an increased risk of transformation to myelofibrosis or acute myeloid leukaemia.9
ELN guidelines recommend switching patients to an alternative cytoreductive therapy from HU if one of the following criteria are met:11,12
HU resistance¶12
Persistent disease-related symptoms: TSS of at least 20 or an itching score of at least 10 for at least 6 months
Persistent thrombocytosis: a platelet count >1000×109 cells/L, microvascular symptoms, or both, persisting for more than 3 months
Symptomatic or progressive splenomegaly: increased in spleen size by more than 5 cm from the left costal margin in 1 year
Progressive and persistent leukocytosis: (at least 100% increase if baseline (BL) count is <10×109 cells/L or at least 50% increase if BL count is >10×109 cells/L) (leukocyte count >15×109 cells/L confirmed at 3 months)
Insufficient HCT control: need for six or more venesections per year to keep HCT<45%
HU intolerance12
Grade 3–4 or prolonged Grade 2 non-haematological toxicity (e.g., mucocutaneous manifestations, gastrointestinal symptoms, fever or pneumonitis) at any dose
Haematological toxicity (Hb <100 g/L, platelet count <100×109 cells/L, or neutrophil count <1×109 cells/L) at the lowest dose of HU to achieve a response
Development of non-melanoma skin cancers
Development of vascular events: either clinically relevant bleeding, venous thrombosis or arterial thrombosis
In patients treated with HU who require a therapy change, either ruxolitinib or interferon alfa should be chosen on the basis of individual clinical features – in particular, age, spleen size, symptoms, history of skin cancers, and patient preferences.‖12
*Data from patients with MPNs (ET, n=302; MF, n=174; PV, n=223) surveyed in the International MPN Landmark Survey (N=699).3
†Data from patients with an MPN (N=286) surveyed in the UK MPN Landmark Survey.4
‡Data from patients with PV (n=78) surveyed in the UK MPN Landmark Survey (N=286).4
§HU resistance or intolerance was defined using the 2010 ELN criteria.7,10 In a retrospective analysis of 106 patients with PV in Belgium, when using the original and modified ELN criteria, 20.7% and 39.6% of patients were resistant or intolerant to HU, respectively.10,11
¶HU dosed at ≥1.5 g per day for at least 4 months and without reporting intolerance.12
‖In particular, age, spleen size, symptoms, history of skin cancers and patient preferences.12
JAKAVI® (ruxolitinib) demonstrated superior haematocrit control and spleen size reduction (composite primary endpoint) vs BAT in the RESPONSE trial**1,13
The RESPONSE trial is a Phase III, randomised, open-label, multicentred study to evaluate the safety and efficacy of JAKAVI® vs BAT in venesection-dependent patients with splenomegaly who have HU-resistant or intolerant PV.13 The composite primary endpoint was HCT control and ≥35% reduction in spleen volume at Week 32.
RESPONSE-2 is a Phase IIIb, prospective, randomised, open-label, multicentred study assessing the safety and efficacy of JAKAVI® vs best available therapy in venesection-dependent patients without splenomegaly who are resistant to or intolerant of HU.14 The primary endpoint was the proportion of patients who achieved HCT control at Week 28.
Study design3,13–15
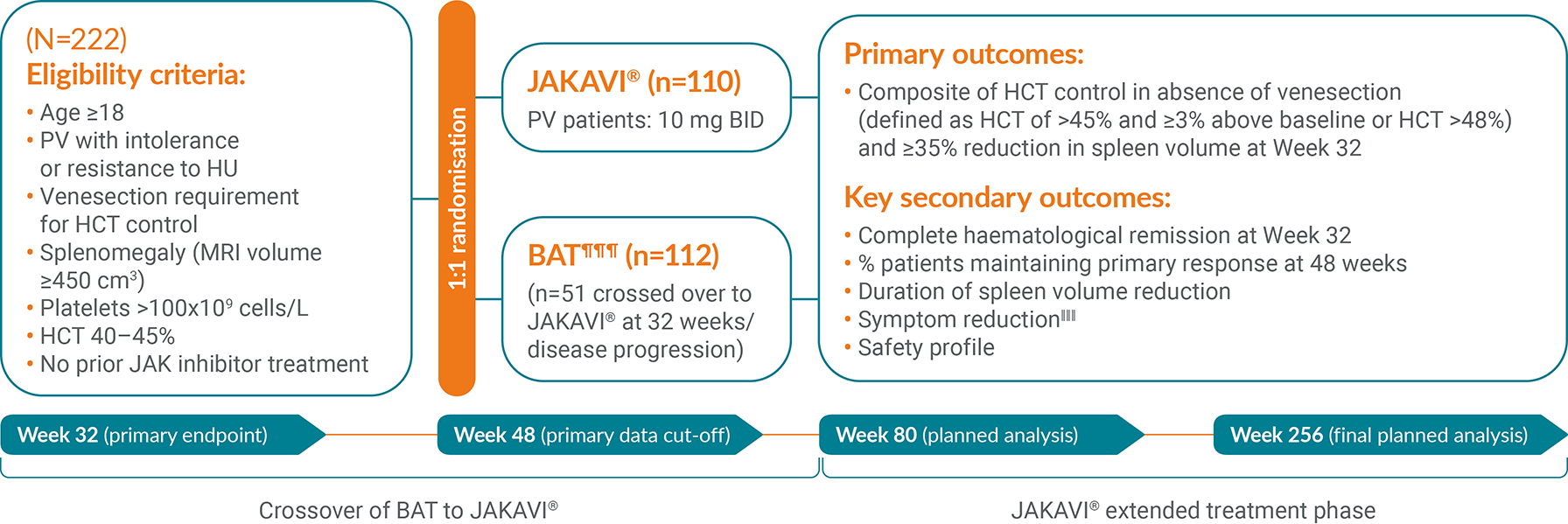
¶¶¶HU, interferon, pipobroman (not licensed for PV in the UK), anagrelide (not licensed for PV in the UK),20 immunomodulators only.15
‖‖‖Measured using MPN-SAF TSS.13
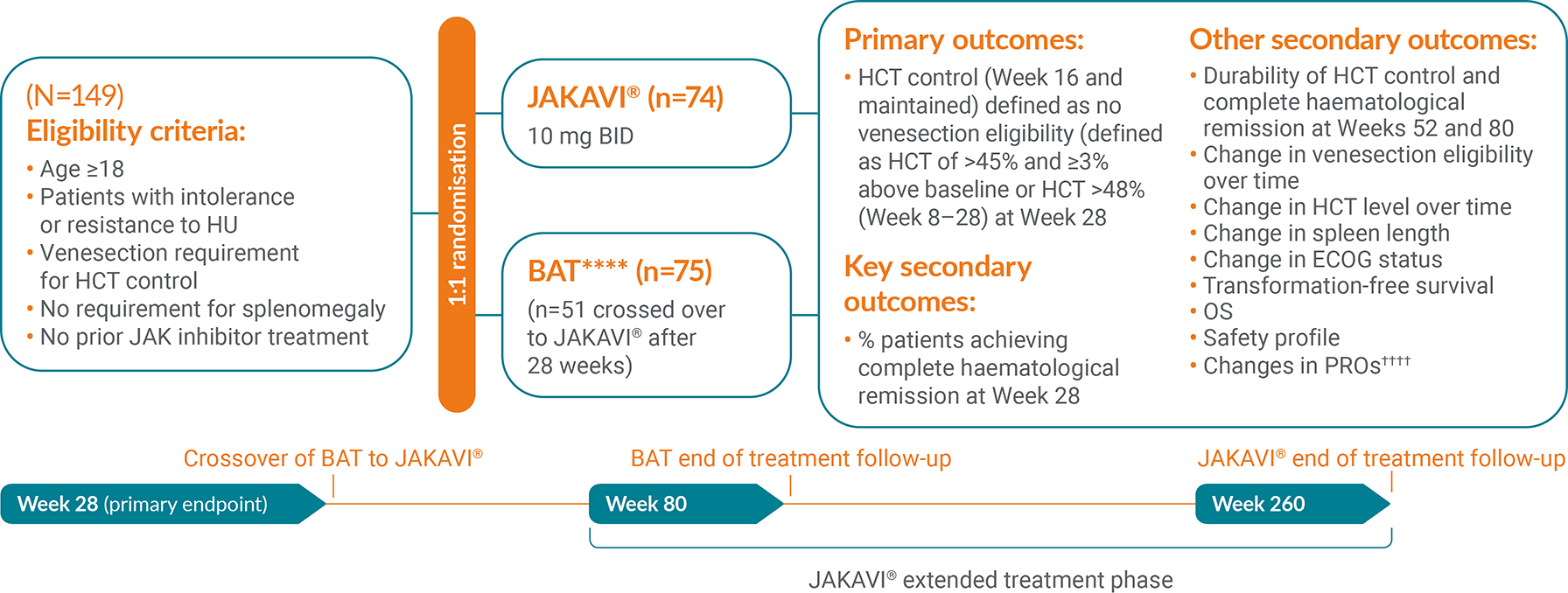
****HU, interferon, immunomodulators and others.16
††††Measured using MPN-SAF TSS.16
More patients achieved HCT control and ≥35% reduction in spleen size with JAKAVI® vs BAT in the RESPONSE trial**13
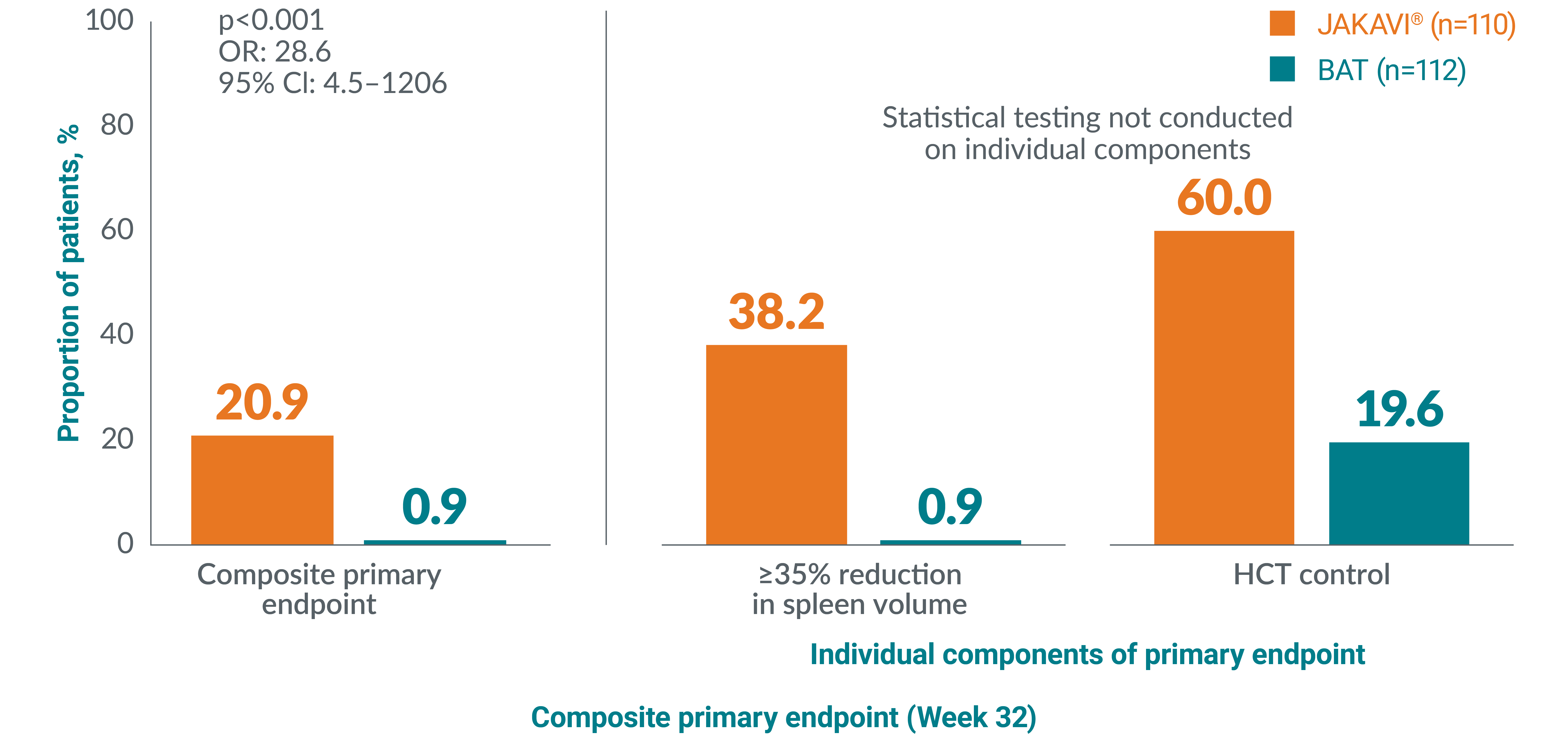
Adapted from Vannucchi AM, et al. 2015.13
Planned analysis after all patients completed 256 weeks (~5 years) of treatment or had discontinued from the RESPONSE study.
At Week 224:
Patients receiving BAT could cross over to JAKAVI after Week 32. It was therefore impossible to compare results at Week 224 to BAT due to crossover.
**HCT control defined as no venesection eligibility from Week 8, with venesection eligibility occurring only once after randomisation and before Week 8. Venesection eligibility was defined as HCT>45% and at least 3 percentage points higher than baseline or HCT >48%.13
††In RESPONSE, patients receiving BAT could cross over to JAKAVI® after Week 32. It was therefore impossible to compare results at 5 years to BAT due to crossover.15
A greater proportion of patients achieved HCT control** at Week 28 with JAKAVI® vs best available therapy in the RESPONSE-2 trial:16
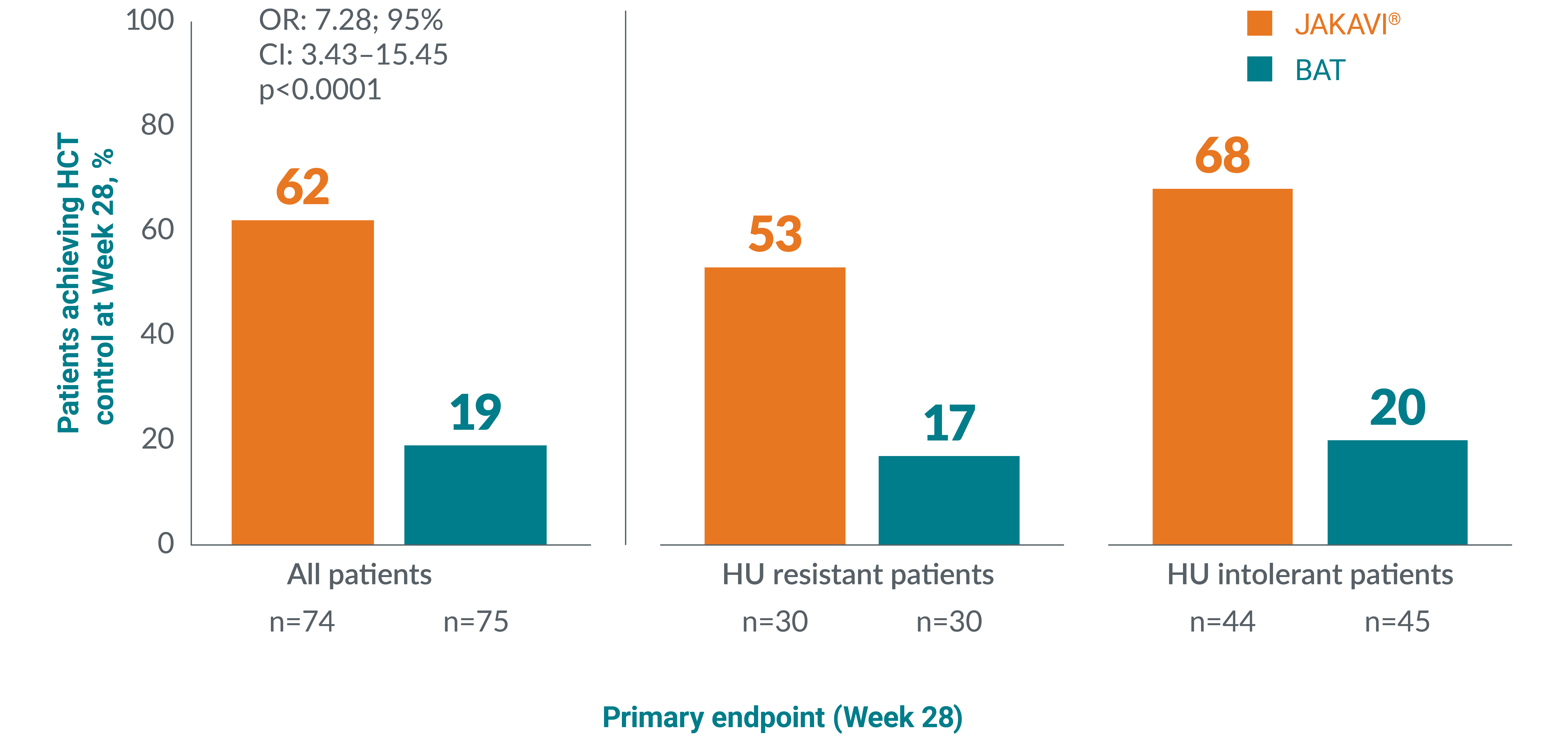
Adapted from Passamonti F, et al. 2017.16
RESPONSE-2 results after all patients completed 260 weeks (~5 years) of treatment or discontinued from the study before Week 260.
22% of patients receiving JAKAVI® achieved and sustained HCT control** through 5 years (n=16/74) vs 0% of patients receiving BAT:17
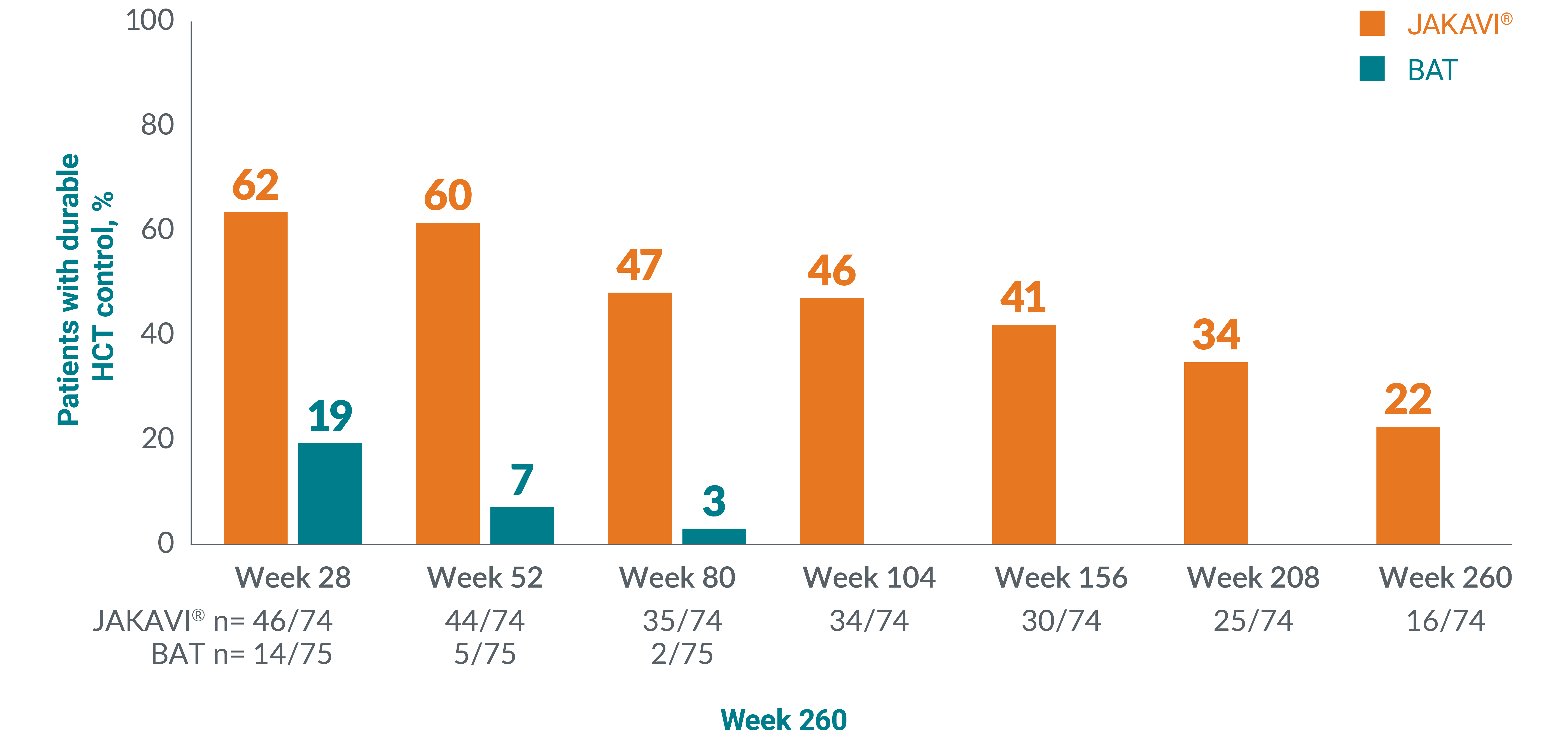
Adapted from Passamonti F, et al. 2022.17
Patients receiving BAT could cross over to JAKAVI after Week 28. No patients continued BAT after Week 80.
**HCT control defined as no venesection eligibility from Week 8, with venesection eligibility occurring only once after randomisation and before Week 8. Venesection eligibility was defined as HCT>45% and at least 3 percentage points higher than baseline or HCT >48%.13
JAKAVI® was associated with observed relief of PV symptoms vs BAT‡‡13
Patients with PV reported numerically greater reductions in MPN-SAF total symptom score and across all symptom clusters with JAKAVI® vs BAT.§§13
No statistical testing performed. Result is observational only.
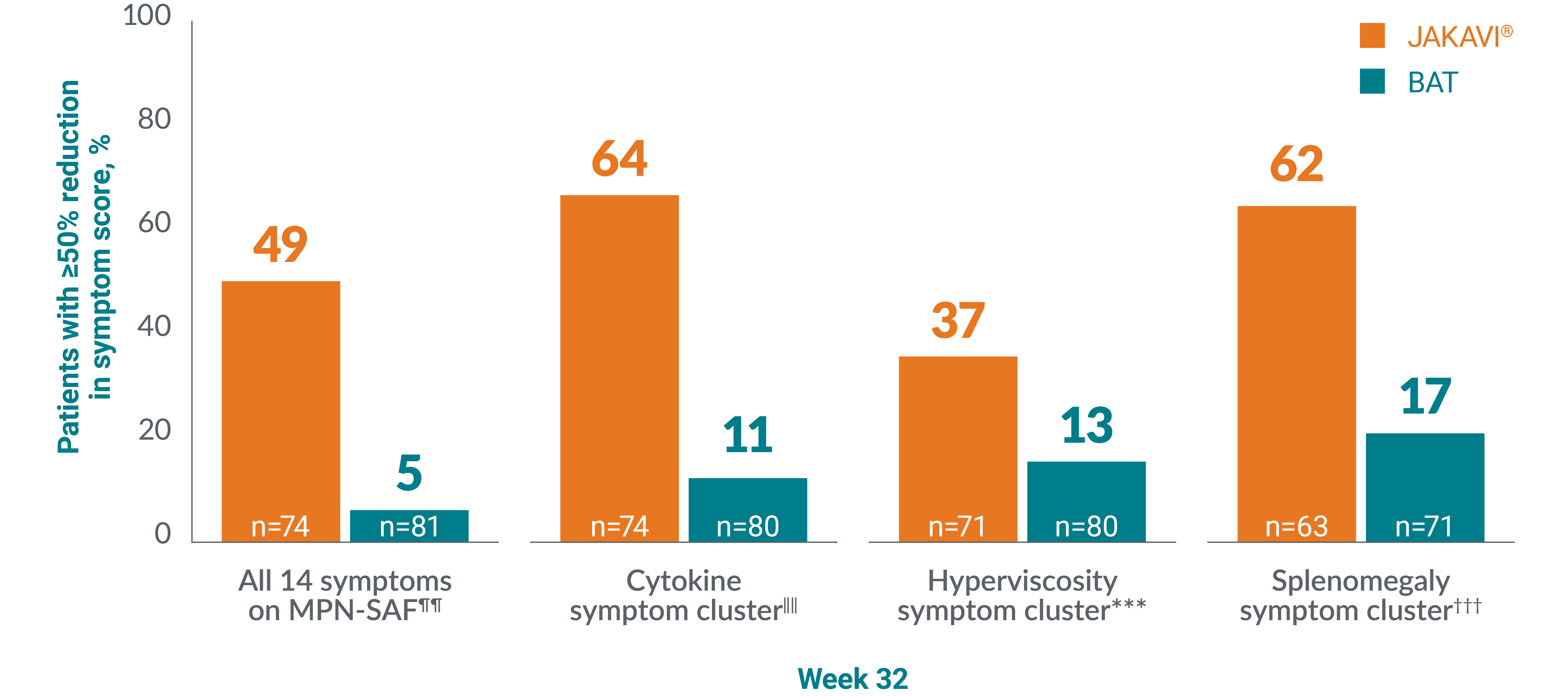
Adapted from Vannucchi AM, et al. 2015.13
‡‡Assessed using MPN-SAF TSS.1,18 p-value not reported.13,19
§§The primary composite endpoint was the proportion of patients achieving both an absence of venesection eligibility (HCT control) and a ≥35% reduction in spleen volume from baseline at Week 32. Venesection eligibility was defined as a confirmed HCT of >45%, i.e. at least 3 percentage points higher than the HCT obtained at baseline or a confirmed HCT of >48%, depending on which was lower. Patients with data at both baseline (value >0) and Week 32 were included in the analysis.13
¶¶Proportion of patients with ≥50% reduction in overall MPN-SAF TSS at Week 32.13
‖‖Proportion of patients with ≥50% reduction in total score for tiredness, itching, muscle ache, night sweats, and sweating while awake at Week 32.13
***Proportion of patients with ≥50% reduction in total score for vision problems, dizziness, concentration problems, headache, numbness or tingling in the hands or feet, ringing in the ears, and skin redness at Week 32.13
†††Proportion of patients with ≥50% reduction in total score for abdominal discomfort and early satiety at Week 32.13
More patients with PV reported complete resolution of symptoms with JAKAVI® vs BAT as well as reported ≥50% reduction from baseline in MPN-SAF total symptom score vs BAT at the end-of-treatment visit:‡‡‡16,17
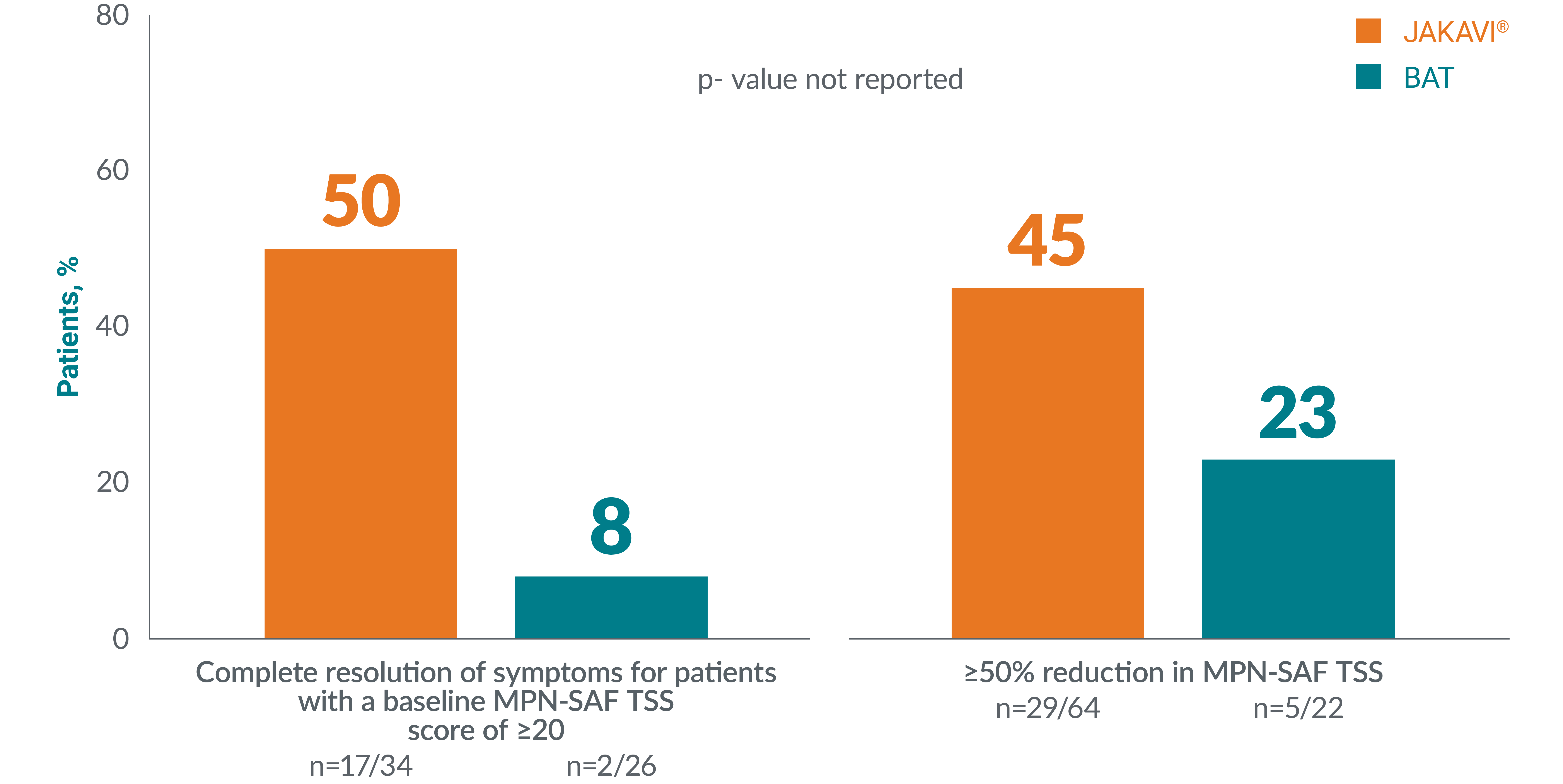
Adapted from Passamonti F, et al. 2017.16
Higher rates of complete haematological remission were also achieved with JAKAVI® vs BAT.§§§13,14,16
RESPONSE: CHR was achieved in 24% of patients in the JAKAVI® group vs 9% in the BAT group (p=0.003).
RESPONSE 2: CHR was achieved in 23% of patients in the JAKAVI® vs 5% in the BAT group (OR 5.58 [95% CI 1.73–17.99], p=0.0019).
‡‡‡Complete resolution of symptoms is defined as MPN-SAF TSS reduction of ≥10 points from baseline at Week 16 and maintained until Week 28 (for patients with a baseline score of ≥20). Complete resolution of symptoms is defined as MPN-SAF TSS reduction of ≥10 points from baseline at Week 16 and maintained until Week 28 (for patients with a baseline score of ≥20).17
§§§CHR is defined as: HCT <45% without venesection for 3 months; platelets ≤400×109/L; WBC count ≤10×109/L.13,16
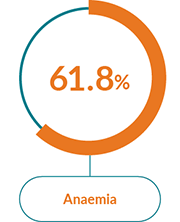
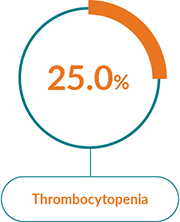
JAKAVI® has a well-characterised safety profile1
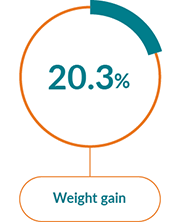
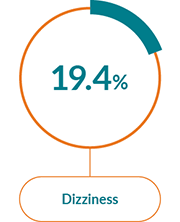
The most commonly reported AEs with JAKAVI® in PV were:1
Haematological AEs
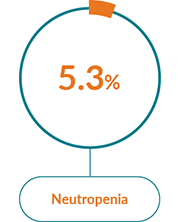
Non-haematological AEs

Very common (≥1/10) | Common | Uncommon (≥1/1,000 to <1/100) | Not known | |
Infections and infestations |
|
|
|
|
Blood and lymphatic system disorders |
|
|
|
|
Metabolism and nutrition disorders |
|
|
|
|
Nervous system disorders |
|
|
|
|
Gastrointestinal disorders |
|
|
|
|
Hepatobiliary disorders |
|
|
|
|
Vascular disorders |
|
|
|
|
Adapted from JAKAVI® Summary of Product Characteristics.1
aADR derived from post-marketing experience.1
bFrequency is based on new or worsened laboratory abnormalities compared to baseline.1
cCommon Terminology Criteria for Adverse Events (CTCAE) version 3.0; grade 1 = mild, grade 2 = moderate, grade 3 = severe, grade 4 = life-threatening.1
dPancytopenia is defined as haemoglobin level <100 g/L, platelet count <100x109/L, and neutrophil count <1.5x109/L (or low white blood cell count of grade 2 if neutrophil count is missing), simultaneously in the same lab assessment.1
Contraindications1
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 of the SmPC
Pregnancy and lactation
Special warnings and precautions1
Myelosuppression
Treatment with JAKAVI® can cause haematological adverse drug reactions, including thrombocytopenia, anaemia and neutropenia. A complete blood count, including a white blood cell count differential, must be performed before initiating therapy with JAKAVI®. Treatment should be discontinued in patients with platelet count less than 50,000/mm3 or absolute neutrophil count less than 500/mm3 (see section 4.2 of the SmPC).
It has been observed that MF patients with low platelet counts (<200,000/mm3) at the start of therapy are more likely to develop thrombocytopenia during treatment.
Thrombocytopenia is generally reversible and is usually managed by reducing the dose or temporarily withholding JAKAVI® (see sections 4.2 and 4.8 of the SmPC). However, platelet transfusions may be required as clinically indicated.
Patients developing anaemia may require blood transfusions. Dose modifications or interruption for patients developing anaemia may also need to be considered.
Patients with a haemoglobin level below 10.0 g/dl at the beginning of the treatment have a higher risk of developing a haemoglobin level below 8.0 g/dl during treatment compared to patients with a higher baseline haemoglobin level (79.3% versus 30.1%). More frequent monitoring of haematology parameters and of clinical signs and symptoms of JAKAVI®-related adverse drug reactions is recommended for patients with baseline haemoglobin below 10.0 g/dl.
Neutropenia (absolute neutrophil count <500) was generally reversible and was managed by temporarily withholding JAKAVI® (see sections 4.2 and 4.8 of the SmPC).
Complete blood counts should be monitored as clinically indicated and dose adjusted as required (see sections 4.2 and 4.8 of the SmPC).
Infections
Serious bacterial, mycobacterial, fungal, viral and other opportunistic infections have occurred in patients treated with JAKAVI®. Patients should be assessed for the risk of developing serious infections. Physicians should carefully observe patients receiving JAKAVI® for signs and symptoms of infections and initiate appropriate treatment promptly. Treatment with JAKAVI® should not be started until active serious infections have resolved.
Tuberculosis has been reported in patients receiving JAKAVI®. Before starting treatment, patients should be evaluated for active and inactive (‘latent’) tuberculosis, as per local recommendations. This can include medical history, possible previous contact with tuberculosis, and/or appropriate screening such as lung x-ray, tuberculin test and/or interferon-gamma release assay, as applicable. Prescribers are reminded of the risk of false negative tuberculin skin test results, especially in patients who are severely ill or immunocompromised.
Hepatitis B viral load (HBV-DNA titre) increases, with and without associated elevations in alanine aminotransferase and aspartate aminotransferase, have been reported in patients with chronic HBV infections taking JAKAVI®. It is recommended to screen for HBV prior to commencing treatment with JAKAVI®. Patients with chronic HBV infection should be treated and monitored according to clinical guidelines.
Herpes zoster
Physicians should educate patients about early signs and symptoms of herpes zoster, advising that treatment should be sought as early as possible.
Progressive multifocal leukoencephalopathy
Progressive multifocal leukoencephalopathy (PML) has been reported with JAKAVI® treatment. Physicians should be particularly alert to symptoms suggestive of PML that patients may not notice (e.g., cognitive, neurological or psychiatric symptoms or signs). Patients should be monitored for any of these new or worsening symptoms or signs, and if such symptoms/signs occur, referral to a neurologist and appropriate diagnostic measures for PML should be considered. If PML is suspected, further dosing must be suspended until PML has been excluded.
Lipid abnormalities/elevations
Treatment with JAKAVI® has been associated with increases in lipid parameters including total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides. Lipid monitoring and treatment of dyslipidaemia according to clinical guidelines is recommended.
Major adverse cardiac events (MACE)
In a large randomised active-controlled study of tofacitinib (another JAK inhibitor) in rheumatoid arthritis patients 50 years of age and older with at least one additional cardiovascular risk factor, a higher rate of MACE, defined as cardiovascular death, non-fatal myocardial infarction (MI) and non-fatal stroke, was observed with tofacitinib compared to tumour necrosis factor (TNF) inhibitors. MACE have been reported in patients receiving JAKAVI®. Prior to initiating or continuing therapy with JAKAVI®, the benefits and risks for the individual patient should be considered particularly in patients 65 years of age and older, patients who are current or past long-time smokers, and patients with a history of atherosclerotic cardiovascular disease or other cardiovascular risk factors.
Thrombosis
In a large randomised active-controlled study of tofacitinib (another JAK inhibitor) in rheumatoid arthritis patients 50 years of age and older with at least one additional cardiovascular risk factor, a dose-dependent higher rate of venous thromboembolic events (VTE) including deep venous thrombosis (DVT) and pulmonary embolism (PE) was observed with tofacitinib compared to TNF inhibitors.
Events of deep venous thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients receiving JAKAVI®. In patients with MF and PV treated with JAKAVI® in clinical studies, the rates of thromboembolic events were similar in JAKAVI® and control-treated patients.
Prior to initiating or continuing therapy with JAKAVI®, the benefits and risks for the individual patient should be considered, particularly in patients with cardiovascular risk factors (see section 4.4 of the SmPC) and additional risk factors for VTE such as history of VTE, major surgery, immobilisation, use of combined hormonal contraceptives or hormone replacement therapy, and inherited coagulation disorder.
Patients with symptoms of thrombosis should be promptly evaluated and treated appropriately.
Second primary malignancies
In a large randomised active-controlled study of tofacitinib (another JAK inhibitor) in rheumatoid arthritis patients 50 years of age and older with at least one additional cardiovascular risk factor, a higher rate of malignancies, particularly lung cancer, lymphoma, and non-melanoma skin cancer (NMSC), was observed with tofacitinib compared to TNF inhibitors.
Lymphoma and other malignancies have been reported in patients receiving JAK inhibitors, including JAKAVI®.
Non-melanoma skin cancers (NMSCs), including basal cell, squamous cell, and Merkel cell carcinoma, have been reported in patients treated with ruxolitinib. Most of the MF and PV patients had histories of extended treatment with hydroxyurea and prior NMSC or pre-malignant skin lesions. Periodic skin examination is recommended for patients who are at increased risk for skin cancer.
Special populations
Renal impairment
The starting dose of JAKAVI® should be reduced in patients with severe renal impairment. For patients with end-stage renal disease on haemodialysis the starting dose should be based on platelet counts for MF patients, while the recommended starting dose is a single dose of 10 mg for PV patients (see section 4.2 of the SmPC). Subsequent doses (single dose of 20 mg or two doses of 10 mg given 12 hours apart in MF patients; single dose of 10 mg or two doses of 5 mg given 12 hours apart in PV patients) should be administered only on haemodialysis days following each dialysis session. Additional dose modifications should be made with careful monitoring of safety and efficacy (see sections 4.2 and 5.2 of the SmPC).
Hepatic impairment
The starting dose of JAKAVI® should be reduced by approximately 50% in MF and PV patients with hepatic impairment. Further dose modifications should be based on the safety and efficacy of the medicinal product. In GvHD patients with hepatic impairment not related to GvHD, the starting dose of JAKAVI® should be reduced by approximately 50% (see sections 4.2 and 5.2 of the SmPC).
Interactions
If JAKAVI® is to be co-administered with strong CYP3A4 inhibitors in MF and PV patients or dual inhibitors of CYP3A4 and CYP2C9 enzymes (e.g. fluconazole) in MF, PV and GvHD patients, the unit dose of JAKAVI® should be reduced by approximately 50%, to be administered twice daily (for monitoring frequency see sections 4.2 and 4.5 of the SmPC).
The concomitant use of cytoreductive therapies with JAKAVI® was associated with manageable cytopenias (see section 4.2 of the SmPC for dose modifications during cytopenias).
Withdrawal effects
Following interruption or discontinuation of JAKAVI®, symptoms of MF may return over a period of approximately one week. There have been cases of patients discontinuing JAKAVI® who experienced severe adverse events, particularly in the presence of acute intercurrent illness. It has not been established whether abrupt discontinuation of JAKAVI® contributed to these events. Unless abrupt discontinuation is required, gradual tapering of the dose of JAKAVI® may be considered, although the utility of the tapering is unproven.
Excipients
JAKAVI® contains lactose. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicinal product.
This medicinal product contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially ‘sodium-free’.
JAKAVI® dosing in PV1
JAKAVI® treatment should only be initiated by a physician experienced in the administration of anti-cancer medicinal products.
Initiation and dosing in polycythaemia vera patients differ from that in myelofibrosis:1

If efficacy is considered insufficient and blood counts are adequate, doses may be increased by a maximum of 5 mg twice daily, up to the maximum dose of 25 mg twice daily.1
The starting dose should not be increased within the first 4 weeks of treatment and thereafter no more frequently than at 2-week intervals.1
In PV, treatment should also be interrupted when haemoglobin is below 8 g/dl. After recovery of blood counts above these levels, dosing may be restarted at 5 mg twice daily and gradually increased based on careful monitoring of CBC, including a WBC differential.1
Discontinue JAKAVI® after 6 months if there has been no reduction in spleen size or improvement in symptoms since treatment initiation1
ADR, adverse drug reaction; AE, adverse event; BAT, best available therapy; BID, twice a day; BL, baseline; CBC, complete blood count; CTCAE, common terminology criteria for adverse events; ECOG, Eastern Cooperative Oncology Group; ELN, European LeukemiaNet; ET, essential thrombocythaemia; HCT, haematocrit; HU hydroxyurea; IFN, interferon; JAK Janus kinase; MACE, major adverse cardiac events; MF, myelofibrosis; MPN, myeloproliferative neoplasm; MPN-SAF TSS, symptom assessment form total symptom score; MRI, magnetic resonance imaging; OS, overall survival; PRO, patient-reported outcome; PV, polycythaemia vera; QoL, quality of life; ULN, upper limit normal; WBC, white blood count.
For further information please consult the Summary of Product Characteristics.
References
JAKAVI® (ruxolitinib) Summary of Product Characteristics.
Tefferi A & Barbui T. Am J Hematol 2020;95:1599–1613.
Harrison CN, et al. Ann Hematol 2017;96:1653–1665.
Harrison CN, et al. OR4-020. BSH 2017, 27–29 March; Brighton, UK.
Novaritis Data on File. Patient Quotes in PV from Advisory Board [MPN_PV_2020_001]. November 2020.
McMullin F, et al. Br J Haematol 2019;184:176–191
Raman I, et al. Leuk Lymphoma 2021;62:2310–2319.
Alvarez-Larrán, et al. Blood 2012;119:1363–1369.
Alvarez-Larrán, et al. Br J Haemtol 2016;172:789–793.
Demuynck T, et al. Ann Hematol 2019;98:1421–1426.
McMullin F, et al. Br J Haematol 2016;172:337–349.
Marchetti M, et al. Lancet Haematol 2022;9:e301–e311.
Vannucchi AM, et al. N Engl J Med 2015;372:426–435.
Griesshammer M, et al. Ann Hematol 2018;97:1591–1600.
Kiladjian JJ, et al. Lancet Haematol 2020;7:e226–e237.
Passamonti F, et al. Lancet Oncol 2017;18:88–99.
Passamonti F, et al. Lancet Haematol 2022;9:e480–92.
Scottish Medicines Consortium. Ruxolitinib (Jakavi). Available at: https://www.scottishmedicines.org.uk/medicines-advice/ruxolitinib-jakavi-full-smc2213/. [Accessed April 2025].
Verstovsek S, et al. Haematologica 2016;101:821–829.
Mylan. Anagrelide summary of product characteristics. Available at: https://www.medicines.org.uk/emc/product/9255/smpc/print [Accessed April 2025].
UK | April 2025 | FA-11208788-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.