

Safety profile in myelofibrosis (MF)
JAKAVI® (ruxolitinib) is indicated in the treatment of disease-related splenomegaly or symptoms in adult patients with primary myelofibrosis (also known as chronic idiopathic myelofibrosis), post polycythaemia vera myelofibrosis or post essential myelofibrosis; the treatment of adult patients with polcythaemia vera (PV) who are resistant to or intolerant of hydroxyurea; the treatment of patients aged 12 years and older with acute graft versus host disease (GvHD) who have inadequate response to corticosteroids; the treatment of patients aged 12 years and older with chronic GvHD who have inadequate response to cortcicosteroids.1
For full information on the safety profile of JAKAVI®, please refer to the Summary of Product Characteristics (SmPC). Click here for the SmPC.
For more information about JAKAVI® in PV, click here.
JAKAVI® has a well-characterised safety profile, with generally manageable AEs1
The safety profile of JAKAVI® in myelofibrosis (MF) has been established across 5-year follow-up to the COMFORT-I and COMFORT-II trials and in real-world studies.1–3
Frequency category of adverse drug reaction reported in MF patients in COMFORT-I and COMFORT-II1
| Very common | Common | Uncommon | Not known |
Infections and infestations |
|
| Tuberculosis | HBV reactivation* |
Blood and lymphatic system disorders† |
|
|
|
|
Metabolism and nutrition disorders |
|
|
|
|
Nervous system disorders |
|
|
|
|
Gastrointestinal disorders |
|
|
|
|
Hepatobiliary disorders |
|
|
|
|
Vascular disorders |
|
|
|
|
Adapted from JAKAVI® Summary of Product Characteristics.1
*ADR derived from post-marketing experience.1
†Frequency is based on new or worsened laboratory abnormalities compared to baseline.1
‡Common Terminology Criteria for Adverse Events (CTCAE) version 3.0; grade 1 = mild, grade 2 = moderate, grade 3 = severe, grade 4 = life-threatening.1
§Pancytopenia is defined as haemoglobin level <100 g/L, platelet count <100x109/L, and neutrophil count <1.5x109/L (or low white blood cell count of grade 2 if neutrophil count is missing), imultaneously in the same lab assessment.1
ADR, adverse drug reaction; AE, adverse event; BAT, best available therapy; BCSH, British Committee for Standards in Haematology; COMFORT, Controlled Myelofibrosis Study with Oral JAK Inhibitor Treatment; CTCAE, common terminology criteria for adverse events; DNA, deoxyribonucleic acid; EPO, erythropoietin; ESA, erythropoietin-stimulating agents; ET, essential thrombocythaemia; GvHD, graft versus host disease; HBV, hepatitis B; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MF, myelofibrosis; NMSC, non-melanoma skin cancer; PML, progressive multifocal leukoencephalopathy; PV, polycythaemia vera; SmPC, summary of product characteristics; ULN, upper limit of normal.
Summary of the safety profile
The most frequently reported adverse drug reactions were thrombocytopenia and anaemia1
Haematological adverse drug reactions (any CTCAE grade) included anaemia (83.8%), thrombocytopenia (80.5%) and neutropenia (20.8%)1
Anaemia, thrombocytopenia and neutropenia are dose-related effects1
The three most frequent non-haematological adverse drug reactions were bruising (33.3%), other bleeding (including epistaxis, post-procedural haemorrhage and haematuria) (24.3%) and dizziness (21.9%)1
Discontinuation due to adverse events, regardless of causality, was observed in 30.0% of patients1
The three most frequent non-haematological laboratory abnormalities identified as adverse reactions were increased alanine aminotransferase (40.7%), increased aspartate aminotransferase (31.5%) and hypertriglyceridaemia (25.2%). In Phase III clinical studies in MF, neither CTCAE grade 3 or 4 hypertriglyceridaemia or increased aspartate aminotransferase, nor CTCAE grade 4 increased alanine aminotransferase or hypercholesterolaemia were observed1
Please refer to the JAKAVI (ruxolitinib) Summary of Product Characteristics for full safety information.1
Contraindications1
Hypersensitivity to the active substance or to any of the excipients
Pregnancy and lactation
Special warnings and precautions1
Myelosuppression:
Treatment with JAKAVI® can cause haematological adverse drug reactions, including thrombocytopenia, anaemia and neutropenia. A complete blood count, including a white blood cell count differential, must be performed before initiating therapy with JAKAVI®. Treatment should be discontinued in patients with platelet count less than 50,000/mm3 or absolute neutrophil count less than 500/mm3.
It has been observed that MF patients with low platelet counts (<200,000/mm3) at the start of therapy are more likely to develop thrombocytopenia during treatment.
Thrombocytopenia is generally reversible and is usually managed by reducing the dose or temporarily withholding JAKAVI®. However, platelet transfusions may be required as clinically indicated.
Patients developing anaemia may require blood transfusions. Dose modifications or interruption for patients developing anaemia may also need to be considered.
Patients with a haemoglobin level below 10.0 g/dL at the beginning of the treatment have a higher risk of developing a haemoglobin level below 8.0 g/dL during treatment compared to patients with a higher baseline haemoglobin level (79.3% versus 30.1%). More frequent monitoring of haematology parameters and of clinical signs and symptoms of JAKAVI®-related adverse drug reactions is recommended for patients with baseline haemoglobin below 10.0 g/dL.
Neutropenia (absolute neutrophil count <500) was generally reversible and was managed by temporarily withholding JAKAVI®.
Complete blood counts should be monitored as clinically indicated and dose adjusted as required.
Infections:
Serious bacterial, mycobacterial, fungal, viral and other opportunistic infections have occurred in patients treated with JAKAVI®. Patients should be assessed for the risk of developing serious infections. Physicians should carefully observe patients receiving JAKAVI® for signs and symptoms of infections and initiate appropriate treatment promptly. Treatment with JAKAVI® should not be started until active serious infections have resolved.
Tuberculosis has been reported in patients receiving JAKAVI®. Before starting treatment, patients should be evaluated for active and inactive (‘latent’) tuberculosis, as per local recommendations. This can include medical history, possible previous contact with tuberculosis, and/or appropriate screening such as lung x-ray, tuberculin test and/or interferon-gamma release assay, as applicable. Prescribers are reminded of the risk of false negative tuberculin skin test results, especially in patients who are severely ill or immunocompromised.
Hepatitis B viral load (HBV-DNA titre) increases, with and without associated elevations in alanine aminotransferase and aspartate aminotransferase, have been reported in patients with chronic HBV infections taking JAKAVI®. It is recommended to screen for HBV prior to commencing treatment with JAKAVI®. Patients with chronic HBV infection should be treated and monitored according to clinical guidelines.
Herpes zoster:
Physicians should educate patients about early signs and symptoms of herpes zoster, advising that treatment should be sought as early as possible.
Progressive multifocal leukoencephalopathy:
Progressive multifocal leukoencephalopathy (PML) has been reported with JAKAVI® treatment. Physicians should be particularly alert to symptoms suggestive of PML that patients may not notice (e.g., cognitive, neurological or psychiatric symptoms or signs). Patients should be monitored for any of these new or worsening symptoms or signs, and if such symptoms/signs occur, referral to a neurologist and appropriate diagnostic measures for PML should be considered. If PML is suspected, further dosing must be suspended until PML has been excluded.
Non-melanoma skin cancer:
Non-melanoma skin cancers (NMSCs), including basal cell, squamous cell, and Merkel cell carcinoma, have been reported in patients treated with ruxolitinib. Most of the MF and PV patients had histories of extended treatment with hydroxyurea and prior NMSC or pre-malignant skin lesions. A causal relationship to ruxolitinib has not been established. Periodic skin examination is recommended for patients who are at increased risk for skin cancer.
Lipid abnormalities/elevations:
Treatment with JAKAVI® has been associated with increases in lipid parameters including total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides. Lipid monitoring and treatment of dyslipidaemia according to clinical guidelines is recommended.
Special populations:
Renal impairment
The starting dose of JAKAVI® should be reduced in patients with severe renal impairment. For patients with end-stage renal disease on haemodialysis the starting dose should be based on platelet counts for MF patients, while the recommended starting dose is a single dose of 10 mg for PV patients. Subsequent doses (single dose of 20 mg or two doses of 10 mg given 12 hours apart in MF patients; single dose of 10 mg or two doses of 5 mg given 12 hours apart in PV patients) should be administered only on haemodialysis days following each dialysis session. Additional dose modifications should be made with careful monitoring of safety and efficacy.
Hepatic impairment
The starting dose of JAKAVI® should be reduced by approximately 50% in MF and PV patients with hepatic impairment. Further dose modifications should be based on the safety and efficacy of the medicinal product. In GvHD patients with hepatic impairment not related to GvHD, the starting dose of JAKAVI® should be reduced by approximately 50%.
Interactions:
If JAKAVI® is to be co-administered with strong CYP3A4 inhibitors in MF and PV patients or dual inhibitors of CYP3A4 and CYP2C9 enzymes (e.g. fluconazole) in MF, PV and GvHD patients, the unit dose of JAKAVI® should be reduced by approximately 50%, to be administered twice daily.
The concomitant use of cytoreductive therapies with JAKAVI® was associated with manageable cytopenias.
Withdrawal effects:
Following interruption or discontinuation of JAKAVI®, symptoms of MF may return over a period of approximately one week. There have been cases of patients discontinuing JAKAVI® who experienced severe adverse events, particularly in the presence of acute intercurrent illness. It has not been established whether abrupt discontinuation of JAKAVI® contributed to these events. Unless abrupt discontinuation is required, gradual tapering of the dose of JAKAVI® may be considered, although the utility of the tapering is unproven.
Excipients:
JAKAVI® contains lactose. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicinal product.
This medicinal product contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially ‘sodium-free’.
Please refer to the JAKAVI (ruxolitinib) Summary of Product Characteristics for further information.1
Consider trial of recombinant EPO in patients with erythropoietin levels <125 units/L
Use 10,000 units 3 times a week or darbepoetin 150 μg once a week (not licensed for anaemia in MF patients)
If no response, double the dose after 1–2 months and discontinue after 3–4 months
Anaemia is the most common haematological side-effect of JAKAVI® treatment, but with appropriate monitoring and dose management, your patients can continue treatment with minimal interruptions.1,4,5
JAKAVI®-induced anaemia is generally manageable2
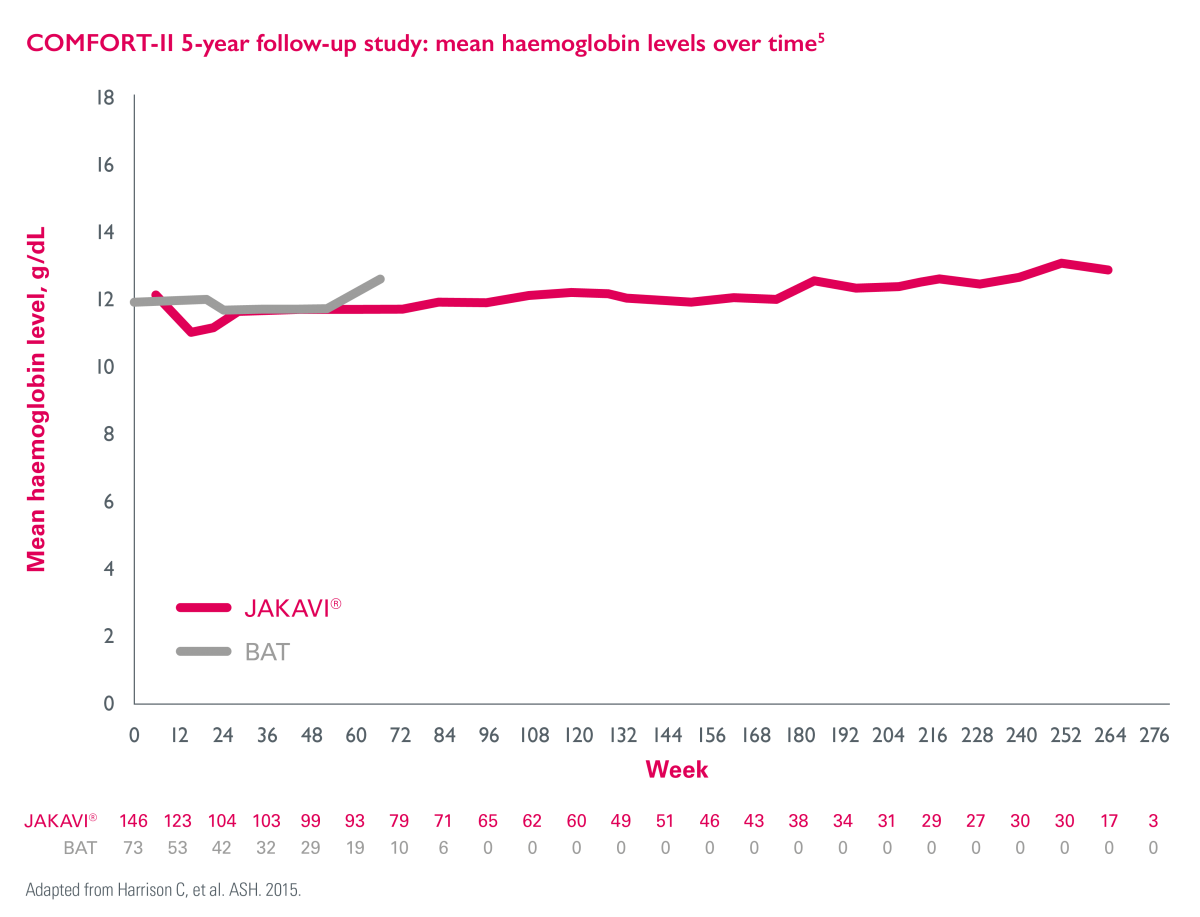
In the JAKAVI® arm, mean haemoglobin levels decreased over the first 12 weeks of treatment and then recovered to levels similar to those in the BAT arm and remained >10 g/dL from Week 24 onward (>151 weeks).5
Please refer to the individual SmPCs for ESA indications.
In a physician survey, it was observed that erythropoietin-stimulating agents (ESAs) are often used first-line to manage treatment-related anaemia if it occurs.7
In a survey of 42 consultant haematologists, 71.4% used ESAs first line.7
The British Committee for Standards in Haematology guidelines recommend the following steps when using ESAs:8
Please refer to the individual SmPCs for ESA indications.
ESAs can be concomitantly prescribed alongside JAKAVI® to manage haemoglobin levels9,10
Footnotes & references
COMFORT-I was a randomised, double-blind, placebo-controlled trial in patients with intermediate-2 or high-risk myelofibrosis. The primary endpoint was the proportion of patients with a reduction in spleen volume of 35% or more at 24 weeks.6
COMFORT-II was a randomised trial comparing JAKAVI® with BAT in patients with primary MF, post-PV MF or post-ET MF. The primary endpoint was the proportion of patients with a reduction in spleen volume of 35% or more at Week 48 and at Week 24.2
The JAKAVI® treatment group consisted of 155 patients in in COMFORT-I and 146 patients in COMFORT-II.
References
JAKAVI® (ruxolitinib) Summary of Product Characteristics.
Harrison C, et al. N Engl J Med 2012;366:787–798.
Verstovsek S, et al. J Hematol Oncol 2017;10:55.
Harrison C, et al. Expert Rev Hematol 2013;6:511–523.
Harrison C, et al. ASH 2015, 5–8 December; Orlando, Florida, USA. Abstract 59.
Verstovsek S, et al. N Engl J Med 2012;366:799–807.
Harrison C, et al. Br J Haematol 2020;188:e80–e112.
Reilly JT, et al. Br J Haematol 2012;158:453–471.
McMullin MF, et al. ASH 2012, 8–11 December; Atlanta, Georgia. Abstract and Poster 2838.
Al-Ali HK, et al. Haematologica 2016;101:1065–1073.
Learn more about MF management in the UK through real-world insights
UK | December 2024 | FA-11317803
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.
